- Volume 68 , Number 3
- Page: 247–57
Single lesion paucibacillary leprosy: baseline profile of the Brazilian multicenter cohort study
ABSTRACT
In Brazil, there is little information about the clinical and epidemiological characteristics of paucibacillary, single skin lesion leprosy patients (SSL-PB). Only recently has the official notification system distinguished leprosy patients with a single lesion as a clinical entity, for whom the singledose ROM (rifampin, ofloxacin and minocycline) regimen has been recommended. In this paper, we describe the baseline clinical features and the immunological background of a multicenter cohort of SSLPB leprosy cases enrolled between December 1997-1998. Patients were recruited at health centers located in the following regions: Southeast = Rio de Janeiro; North = Amazon and Rondônia states and CenterWest = Goiás state. Eligible cases were newly detected, untreated single-lesion leprosy patients without thickened nerve involvement, and were assessed by clinical, bacilloscopic and histopathological exams. The Mitsuda skin test and anti-PGL-I serology (ELISA) were also performed. Of the 299 SSL-PB leprosy patients, 259 (86.6%) fulfilled the criteria for single-dose ROM intervention. Our results showed that patients recruited from different sites had similar features, considering the clinical and immunological profiles. There was a predominance of adults (mean age 32.4; S.D. 16.0), and a BCG scar was detected in 76.7% of the children (<15 years old). Only 7 cases were diagnosed as the multibacillary type, representing less than 3% of the patients being misclassified. Our data indicate that in Brazil SSL-PB case ascertainment based on clinical and bacilloscopic criteria can be accurately defined under a routine control program; 75.0% of SSL-PB cases were Mitsuda positive (>5 mm) and seropositivity for anti-PGL-I was detected in 17.3% of the patients. These data are compatible with effective cell-mediated immunity and low bacillary load, suggesting favorable clinical outcomes for most SSLPB participants of this cohort.RÉSUMÉ
Peu d'informations existent au Brésil en ce qui concerne les caractéristiques cliniques et épidémiologiques des patients qui présentent une lésion cutanée unique paucibacillaire (LCU-PB). Il n'y a que très récemment que le système officiel de dépistage a distingué les patients hanséniens présentant un seule lésion comme une entité clinique distincte, pour lesquels une administration unique de rifampicine, ofioxacine et minocylcine (ROM) a été recommandée. Nous décrivons ici les caractéristiques cliniques et immunologiques d'une cohorte de LCU-PB sélectionnée entre décembre 1997 et décembre 1998. Les patients furent recrutés dans des centres de soins localisés dans les régions suivantes: sud-est = Rio de Janeiro; nord = états de Rondônia et de l'Amazone; centre ouest = état de Gaia. Les cas sélectionnés étaient des patients hanséniens nouvellement détectés, non-traités, présentant une lésion unique sans évidence de lésion d'épaississement des nerfs. Ces cas furent évalués par des examens cliniques, bacilloscopiques et histopathologiques, ainsi que des réactions de Mitsuda et des test sérologiques (ELISA) anti-PGL-I. Parmi 299 patients hanséniens LCU-PB, 259 (86,6%) présentaient les critères pour un traitement unique ROM. Nos résultats montrent que les patients enrôlés à partir de sites différents présentaient des caractères similaires, si l'on considère les profils cliniques et immunologiques. Les patients étaient en majorité adultes (moyenne de 32,4 ans; D.S. = 16,0) et une cicatrice de BCG fut détectée chez 76,7% des enfants (<15 ans). Il n'y eut que 7 cas qui furent diagnostiqués comme étant du type multibacillaire, ce qui ne représente que 3% des patients qui furent re classifies à posteriori. Nos données suggèrent que, au Brésil, l'évaluation des cas LCU-PB basée sur des critères cliniques et bactérioscopiques peut être définie de façon précise dans le cadre d'un programme de contrôle de routine; 75,0% des cas SLC-PB étaient positifs à la réaction de Mitsuda (>5 mm), et 17,3% des patients étaient séropositifs au test anti-PGL-I. Ces données sont compatibles avec une efficace immunité à médiation cellulaire et une charge basse en bacilles, suggérant une issue clinique favorable pour la majorité des patients LCU-PB de cette cohorte.RESUMEN
En Brasil hay poca information sobre las características clínicas y epidemiológicas de los pacientes paucibacilares con una sola lesión dérmica (SSL-PB). Solo recientemente el sistema de notification oficial ha reconocido a los pacientes con lepra con lesiones únicas, como una entidad clínica definida para la cual se ha recomendado el tratamiento con una sola dosis de rifampina-ofloxacina-minociclina (ROM), En este trabajo se describen las características clínicas e inmunológicas de un grupo de pacientes SSL-PB registrados entre Diciembre de 1997 y Diciembre de 1998. Los pacientes fueron reclutados en los centros de salud de los estados de Rio de Janeiro (Sureste), Amazónia y Rondonia (Norte) y Goiás (Centro-oeste). Los casos elegibles fueron casos nuevos, no tratados, con lesiones únicas y sin engrosamiento de nervios valorados por exámen clínico, baciloscópico e histopatológico. En estos pacientes también se realizaron la réaction de Mitsuda y la búsqueda de anticuerpos por ELISA contra el GLP-I. De los 200 pacientes SSL-PB, 259 (86.6%) cumplieron con los critérios necesarios para su tratamiento con ROM. Los resultados mostraron que los pacientes reclutados de diferentes sítios tuvieron características clínicas e inmunológicas similares. Hubo un predomínio de adultos (32.4 ± 16.0 anos) y se detecto una cicatriz de BCG en el 76.7% de los nifios (<15 anos de edad). Sólo 7 casos se diagnosticaron como multibacilares y representaron menos dei 3% de los pacientes mal clasificados. Nuestros datos indican que en Brasil la identificación de casos SSL-PB basada en critérios clínicos y baciloscópicos puede lograrse acertadamente bajo un programa rutinario de control. Setenta y cinco porciento de los casos SSL-PB fueron Mitsuda-positivos (>5 mm), y el 17% de los pacientes tuvieron anticuerpos contra el PGL-I. Estos datos, compatibles con una efectiva inmunidad celular y baja carga bacteriana, sugieren una evolución favorable en la mayoría de los pacientes SSL-PB de esta cohorte.In the context of leprosy elimination, there has been an increased effort to detect and treat leprosy as early as possible with short drug regimens. Single skin lesion paucibacillary leprosy (SSL-PB) may be considered an early chronic stage of the disease, with reported high prevalence in India (5, 6, 26). In Brazil, only recently has the official notification system distinguished leprosy patients with monolesion within the paucibacillary (PB) group (Ministério da Saúde Brasil. Alterações nas instruções normativas do Plano Nacional de Eliminação da Hanseníase. Ofício Circular No. 67, CNDS/CENEPI, 1998). Therefore, epidemiological information regarding patient characteristics is limited to one outpatient clinic in the Southeast Brazilian region (2). Since 1998, the World Health Organization (WHO) has recommended a single-dose ROM therapeutic regimen consisting of rifampin (600 mg), ofloxacin (400 mg) and minocycline (100 mg) based on a multicenter, double-blind field trial conducted among SSL-PB patients in India. In this study, similar cure rates were observed for the ROM regimen when compared to the standard 6-month WHO multidrug therapy (WHO/MDT) (23).
So far the scientific evidence from the available publications on single-dose ROM efficacy has raised many polemic issues concerning both the clinical outcome in long-term follow up and the immune profile among SSL-PB patients (10, 11). Although there is a lack of in-depth investigations about the immune status of this category of patients, a minimal therapy schedule has been recommended under the assumption that this group has low bacterial load, preserved cell-mediated immunity and high spontaneous cure rates. However, it has been argued that single lesion leprosy patients may not comprise a homogeneous group regarding immunological and clinical parameters, and these differences may distinctly influence the clinical prognosis within this category (l4,16).
Recently, the Pan-American Health Organization and the Brazilian Ministry of Health sponsored a multicentric study to implement the ROM therapy for single lesion PB leprosy patients in selected areas of Brazil. This represented a unique opportunity to assess the comparability of an SSLPB cohort recruited in Reference Centers located in different endemic settings.
In this paper we describe the baseline clinical features of patients enrolled in the Brazilian monolesion leprosy cohort study. The predictive value of the clinical screening to diagnose single lesion leprosy and the immunological background of this particular group of patients treated with the one-dose ROM therapy are also presented.
MATERIALS AND METHODS
This study was preceded by the National Dermatologic Campaign launched by the Brazilian Ministry of Health in October 1997 which involved radio, newspaper and television publicity. Announcements were placed in public health centers and in physicians' offices in order to optimize population-based screening for the detection of early leprosy. Before the nationwide implementation of single-dose ROM therapy in the routine attendance of the Leprosy Control Program, we started a standardized surveillance in outpatient clinics for recruiting monolesion PB leprosy patients in three endemic Brazilian regions.
Selection of centers
The requirements for the selection of the sites were to have: a local leprosy control program with expertise in implementing WHO/MDT regimens; an adequately trained staff for clinical exams, reading skin smears and case management; the capability to recruit around 50 monolesion leprosy patients within 12 months; a reasonably equipped base hospital in the area for patient admission in case of severe side effects or reactions that might occur.
SSL-PB patients were recruited from health centers from four Brazilian states located in the following regions (Fig. 1): a) North = "Fundação Alfredo da Matta," a WHO Leprosy Reference Center located in the city of Manaus (1,280,000 inhabitants), Amazon state, and in two health centers in the municipalities of Porto Velho and Ariquemes (300,000 and 70,000 inhabitants, respectively), Rondônia state, b) Southeast = "Fundação Oswaldo Cruz," a National Leprosy Reference Center and outpatient clinic of the University Hospital Clementino Fraga Filho in the city of Rio de Janeiro (5,730,000 inhabitants), Rio de Janeiro state, c) Center-West = Regional reference leprosy outpatient clinic located in the city of Goiânia (1,000,000 inhabitants), Goiás state.
Fig. 1. Brazilian Single-lesion leprosy cohort study cites.
Ascertainment of cases
SSL-PB cases were defined taking into account clinical assessment, bacilloscopy and histopathology. Patients were clinically screened by well-trained doctors in the participating health care centers. Potential eligible cases were newly detected, untreated monolesion leprosy with a skin lesion characterized by hypopigmentation or erythema or both, with or without infiltration, definite sensory loss without thickened nerve involvement. All monolesion cases suspected of leprosy had a skin smear and a skin biopsy for bacilloscopy and conventional histopathologic exams. Patients were categorized as paucibacillary (PB) if they had a negative bacterial index (BI) on slit-skin smear and if the biopsy was classified as indeterminate (I), tuberculoid (TT) or borderline tuberculoid (BT) according to Ridley and Jopling criteria (17).
Criteria for exclusion from the study were: patients with histopathological readings consistent with multibacillary (MB) types of leprosy or with evidence of other skin diseases; children younger than 7 years of age; individuals with sensory loss without a visible skin lesion; pregnant women or those breast feeding at the time of drug intake; known HIV seropositive test; chronic diseases (liver dysfunction, diabetes), or an allergy that might interfere with any of the proposed drugs. The multicenter study design is presented in Figure 2.
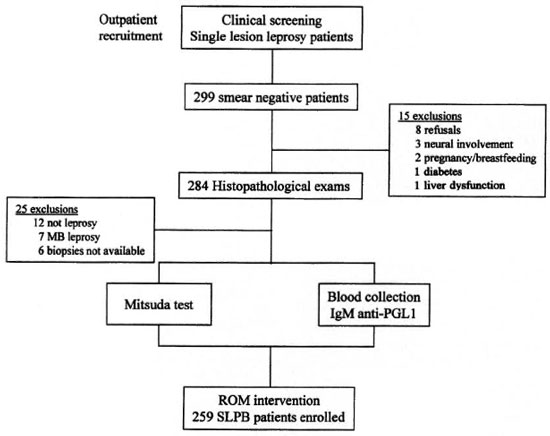
Fig. 2. Multicenter study profile.
Baseline data collection
Clinical features. At the time of enrollment a questionnaire was used to obtain information on age, sex, height, weight, time of lesion perception and intra-domiciliary contacts of leprosy cases. A clinical examination was performed including the number and size of lesion measured by the larger diameter and characteristics of the skin lesion; nerve enlargement; and the presence of a BCG scar. To assess neurological sensory loss on the lesion (anesthesia), thermal, pain sensation and standard monofilament devices were used for all patients. Motor function was also tested to grade disability.
The Mitsuda test was performed by intradermally injecting 0.1 ml of lepromin (same batch, produced in Brazil, 1997) on the volar surface of the forearm. The largest transverse diameter of palpable induration was measured 4 weeks later. A positive Mitsuda test was defined as having an induration of >5 mm as assessed by the clinical staff.
Laboratory tests. A slit-skin smear, skin biopsy and blood samples were taken before ROM therapy. Smears were collected from earlobes, elbows, and the skin lesion, and the BI was determined in each of the participating centers. Skin biopsies were collected, fixed in 10% buffered formalin, and processed for paraffin sections (5 gm).The sections were stained with hematoxylinand eosin (H&E) and Fite-Faraco for acid-fast bacilli (AFB). The histopathological di-agnosis of leprosy was based on Ridley and Jopling criteria (l7). All exams were centrally read by one experienced pathologist at the Federal University of Goiás in Central Brazil. (Details of histopathological findings are out of the scope of this paper.)
Blood samples were collected and serum samples were sent to the Immunology Laboratory of the Federal University of Goiás (UFG), stored at -20°C and tested at once. IgM antibodies specific to Mycobacterium leprae phenolic glycolipid-I (PGL-I) were assayed by ELISA, using PGL-I synthetic disaccharide provided by Dr. M. J. Colston (IMMLEP, London). The laboratory staff were kept blind from the previous clinical, bacteriological and histopathological assessments. The cut-off point for positivity was 0.2 optical density (OD) as described previously (24). Biochemical tests (hemogram, AST, ALT, blood sugar and a pregnancy test) were performed for ROM exclusion criteria purposes.
All eligible patients were treated with the single-dose ROM regimen (25). Children received a half dose according the Brazilian Ministry of Health recommendation. Side effects were actively investigated after drug administration or recorded by passive reporting during the first month of follow up.
Data analysis
The data were entered and analyzed centrally at the Coordinator Center in Goiânia, Central Brazil, where a master file with a copy of all protocols was archived. The baseline characteristics of each participating center were polled by the state of recruitment. The body mass index (BMI) was calculated as weight in kilograms divided by the square of the height in meters for participants older than 13 years of age (22). The differences in the distribution of the variables were tested by the ANOVA or the chi-squared test to compare continuous or categorical variables, respectively. The SPSS/PC program was used for descriptive and exploratory data analysis.
Written informed consent was obtained from all participants, according to the approved protocol in each of the institutions, by the National Human Research Ethical Committee (CONEP) and by the Leprosy Control Program at the national level.
RESULTS
Figure 2 summarizes the screening procedures for selecting the participants from the Brazilian recruitment centers between December 1997 and December 1998. Among patients clinically screened to detect SSL-PB, 299 patients had negative skin smears. Fifteen patients were excluded on clinical/laboratory criteria, and the remaining 284 had skin biopsies taken. Additionally, 25 cases (8.8%) were excluded because either the histopathological results were not consistent with PB leprosy or because a skin biopsy was not available. Leprosy was classified as TT (33.6%), BT (33.6%) and I (29.7%) according to Ridley and Jopling clinico-pathological criteria (17), and 3.1% of the patients were considered to have leprosy by clinical criteria only. Taking histopathology as the gold standard, 12 biopsies were diagnosed as other skin diseases and 7 were considered MB cases, yielding a total of 19 false-positive SSL-PB. Therefore, the positive predictive value of the clinical screening was 93.2% (259/278).
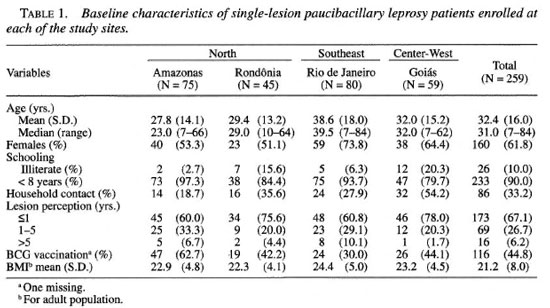
As shown in Table 1, 259 (86.6%) SSLPB patients fulfilled the eligibility criteria for ROM intervention. Patients recruited in the North region accounted for the majority of participants (46.3%), followed by the Southeast (30.9%) and Central-West (22.8%) regions. There was a preponderance of adults, mean age 32.4 (S.D. = 16.0), and patients from the city of Rio de Janeiro were older than patients assembled in the other regions (Ftossi = 7.0; p <0.001). There was a 1:1 male/female ratio in the North region, but in the other regions females represented 66.5% of the adult population and 38.6% of patients younger than 15 years of age. The presence of a BCG scar varied from 30.0% to 62.7% among patients from different sites. Overall, 33.2% of the participants had knowledge of a previous leprosy case in the household; 67.1% of the participants reported that the lesion had been perceived within a time span of less than 1 year. BMI values were within the normal range for patients older than 13 years, and there was no statistically significant difference among regions.
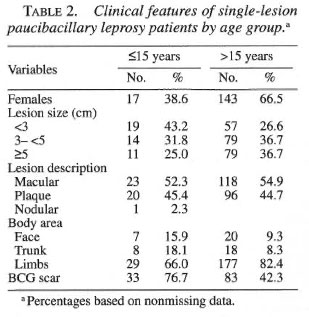
Analysis of the dermatological features showed marked similarity among the sites. Children (<15 years) had smaller lesion size than adults, 3.7 cm (95%CI 3.0-4.5) versus 5.1 cm (95%CI 4.6-5.6), yielding a statistically significant difference. Independent of the age group, most skin lesions were described as a macular hypochromic or erythematous lesion (47.9%) and only one child presented with a nodule on the face. Most of the skin lesions were detected on the limbs of children and adults (Table 2). A BCG scar was detected in 76.7% of the children and in 42.3% of adults (χ2 = 19.5; p <0.001). All patients were considered to have at least moderate sensory loss on the lesion by monofilament evaluation, and no grade of disability was detected.
Figures 3 and 4 show box-plot distributions of the Mitsuda readings and the antiPGL-I serology, respectively. Similar patterns were obtained among patients from different geographical regions: 75.0% of the patients were Mitsuda positive (>5 mm); in 67 patients (24.9%) the induration was smaller than the arbitrary "positive-negative" cut-off Mitsuda response at 5 mm. The prevailing values of anti-PGL-I serology were negative, below the cut-off point (OD of 0.2), among participants from all regions. Only 17.3% of the patients were considered positive for anti-PGL-I antibodies compatible with the low bacillary load of PB patients; 61.0% (142/233) of SSL-PB patients had a simultaneously positive Mitsuda reading and negative anti-PGL-I serology.
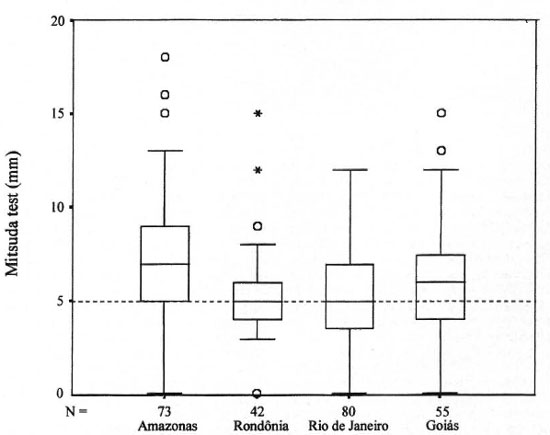
Fig. 3. Box-plot distribution of Mitsuda skin readings among single-lesion paucibacillary leprosy patientsenrolled at each of the study sites. Boxes = 25th and 75th percentiles with a median bar between. O and * = out-liers and extreme values of the distribution. Dashed fine = cut-off value.
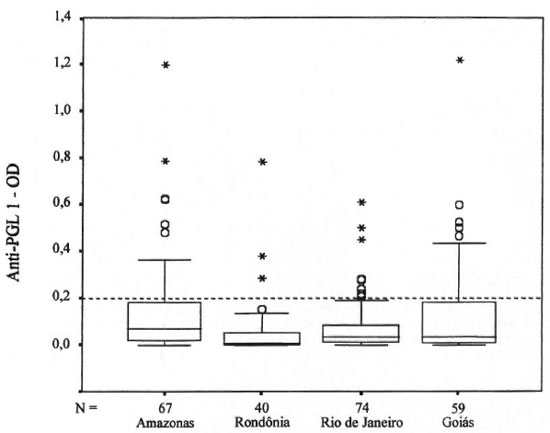
Fig. 4. Box-plot distribution of anti-PGL-I antibodies (OD) among single-lesion paucibacillary leprosy pa-tients enrolled at each of the study sites. Boxes = 25th and 75th percentiles with a median bar between.  and *= outliers and extreme values of the distribution. Dashed line = cut-off value.
and *= outliers and extreme values of the distribution. Dashed line = cut-off value.
The ROM regimen was well tolerated among adults and children. Slight adverse side effects, mainly gastrointestinal symptoms such as nausea, stomach ache, and diarrhea, were reported for approximately 15% of the patients shortly after drug intake. No patient required specific medical attendance for these symptoms.
DISCUSSION
Only recently has single skin lesion been distinguished as a subtype of leprosy in the Brazilian National Control Program following the classification proposed by WHO taking into account the number of lesions (26 and Ministério da saúde Brasil. Alterações nas instruções normativas do Plano Nacional de Eliminação da Hanseníase. Oficio Circular No. 67, CNDS/CENEPI, 1998). The baseline characteristics of the SSL-PB patients enrolled in this Brazilian ROM multicentric study provide a unique and comprehensive picture of the clinical and immunological profile of monolesion leprosy patients in different epidemiological settings. Patients recruited from the different sites had similar features, however, with a common pattern of variability concerning dermatological aspects, size and location of the lesion. Regardless of the endemicity of the recruitment site, patients were mainly adults, a different situation from other studies in which the majority of single-lesion leprosy cases were reported among children (1,3).
In our multicentric study, the majority of patients with monolesion were indeed paucibacillary with no grade of disability, suggesting early leprosy detection. However, among 299 clinically suspected SSL-PB leprosy patients screened, seven MB (2.4%) monolesion cases were detected, in concordance with some case reports of multibacillary monolesion (9,28). A wide variation of dermatological features was observed within SSL-PB patients, but lesions smaller than 5 cm predominated among children and adults and the limbs were the prevailing affected body areas. It has been suggested by other authors that the monolesion can mainly be distributed on body regions exposed to trauma, suggesting the cutaneous surface as a possible portal of entry (8). However, it is outside the scope of this study to make any assumption about the possible transmission routes in leprosy.
Several methodological issues have been raised about previously published single-lesion studies. The main concern refers to case ascertainment and the lack of specificity of an early leprosy diagnosis based solely on clinical evaluation (11,16). Taking into account the lack of an independent gold standard for the diagnosis of the paucibacillary type of leprosy, clinical examination and conventional histopathology still remain the standard for diagnosis in the early chronic stage of the disease for research purposes (l5'21). In our study, clinical, smear-bacilloscopy data and histopathology results were combined in an attempt to improve the specificity of the monolesion leprosy diagnosis.
It is well known that in many settings around the world, including Brazil, clinicians will not have histopathology support for leprosy diagnosis. Considering the casemanagement expertise of our recruitment sites, there was a high positive predictive value of the clinical evaluation (around 90%). Only 19 (12 not leprosy and 7 MB cases) out of 278 patients were excluded when histopathology was taken as the gold standard for early leprosy diagnosis. This represents less than 3% of multibacillary forms among monolesion patients diagnosed using clinical and bacilloscopic criteria in the three endemic Brazilian regions studied. Our observations indicated that in Brazil case ascertainment of SSL-PB can be accurately defined under a routine control program based upon clinical examination and bacilloscopy of skin smears. However, we should keep in mind the difficulties of assessing false-negative or -positive results when only clinically suspected leprosy is submitted to skin biopsy, leading to a possible verification bias (l9). Refined molecular biology tools, such as the detection of M. leprae DNA by polymerase chain reaction, may contribute to improve the specificity of early leprosy diagnosis (4,7,20), but this still remains to be validated under the field conditions of a control program.
Single lesion paucibacillary leprosy has been considered the earliest clinical manifestation of the disease that may undergo self-healing or progress to any form of the disease spectrum, eventually evolving to nerve damage (12). It has been widely accepted that the local cellular immunity dictates the outcome of leprosy, with the Thl CD4+ lymphocyte as the pivotal cell for protection or pathogenesis (l3,27). This is a well-established model for the chronic stage of the disease; however, the usefulness of these markers for prognosis in the early chronic stage of leprosy remains to be demonstrated. Up to now, the Mitsuda test has been one of the most used field tools to assess cellular immunity in leprosy patients, particularly useful in the early stage of disease. In general, our SSL-PB patients had a strong positive response to lepromin with a strikingly similar profile among the recruitment sites, suggesting intact cell-mediated immunity and, therefore, favorable clinical prognosis. Since it is widely accepted that PGL-I positivity reflects bacillary load (18,24), the predominance of seronegative patients is compatible with low bacillary load. Taking the Mitsuda and anti-PGL-I results together, it seems reasonable to envision a favorable outcome for most of the single-lesion participants of this cohort. Only an extended clinical follow up in progress, incorporating additional molecular markers, may provide insights about the importance of the immune profile in the disease prognosis among these early lesion patients.
Acknowledgment. We thank Dr. Euzenir Nunes Sarno, Dr. Maria Leide W. de Oliveira and Dr. David Scollard for helpful suggestions and Dr. M. J. Colston for the PGL-I antigen. We also thank Dr. Uma M. Silva, Dr. Juan Pinero Maceira, Dr. Antonio Pedro M. Schettini, and Dr. Emilia Sayurii Ueda for their help and encouragement in setting up this project. Karen Marcelo de Deus received a fellowship (PIBIC/ CNPq). This study could not be done without the cooperation of the participants. This study was partially sponsored by PA 110the Brazilian Ministry of Health, and FUNAPE-Federal University of Goiás in 1998. This investigation received financial support from the UNDP/World Bank/WHO Special Program for Research and Training in Tropical Diseases (TDR).
REFERENCES
1. Abraham, S., Mozhi, N. M., Joseph, G. A., Kurian, N., Sundar Rao, P. S. S. and Job, C. K. Epidemiological significance of first skin lesion in leprosy. Int. J. Lepr. 66 (1998)131-139.
2. Avelleiria, J. C. R., Vianna, R. E, Coutinho, R. B., Marques Boechat, A. and Gomes de Andrade, V. R. Distribution of single cutaneous lesion in paucibacillary leprosy. Acta Leprol. 8 (1993) 127-131.
3. Bechelli, L. M., Garbajosa, G. P., Gyi. M. G., Dominguez, V. M. and Qlialiato R. Site of early lesions in children with leprosy. Bull. WHO 48 (1973) 107-111.
4. Cho, S.-N. and Brennan, P. J. New biological tools for leprosy surveillance. Int. J. Lepr. 67(1999) S59-S62.
5. Gillis, T. P. and Krahenbuhl, J. L. Global elimination of leprosy. Rev. Med. Microbiol. 9(1998)39-48.
6. Jacobson, R. R. and Krahenbuhl, J. L, Leprosy. Lancet 353(1999)655-659.
7. Job, C. K., Baskaran, B. Jayakumar, J. and Aschhoff, M. Histopathologic evidence to show that indeterminate leprosy may be a primary lesion of the disease. Int. J. Lepr. 65(1997)443-449.
8. Job, C. K., Jayakumar, J., Williams, D. L. and Gillïs, T. P. Role of polymerase chain reaction in the diagnosis of early leprosy. Int. J. Lepr. 65(1997)461-464.
9. Job, C. K., Kahkonen, M. E., Jacobson, R. R. and Hastings, R. C. Single lesion subpolar lepromatous leprosy and its possible mode of origin. Int. J. Lepr 57 (1989)12-19.
10. Katoch, V. M. Is there a microbiological rationale for single-dose treatment of leprosy? Lepr. Rev. 69(1998)2-5.
11. Lockwood, D. N. J. Rifampicin/minocycline and ofloxacin(ROM)for single lesions-What is the evidence? Lepr. Rev. 68(1997)299-300.
12. Lucas, S. Bacterial disease. In: Lever's Histopathology of the Skin. 8th edn. Philadelphia: Lippincott-Raven Publishers, 1997, pp. 477-502.
13. Modlin, R. L,, Melancon-Kaplan, J., Young, S. M. M., Pirmez, C., Kino, H., Convit, J., Rea, T. H. and Bloom, B. R. Learning from lesions: patterns of tissue inflammation in leprosy. Proc. Natl. Acad. Sci. U.S.A. 85(1988)1213-1217.
14. Opromolla, D. V. A. Single lesion leprosy. Hansen. Int. 21 (1996)1-2.
15. Pannikar, V, K. Defining a case of leprosy. Lepr. Rev. 63(1992)61s-65s.
16. Ponnighaus, J. M. Diagnosis and management of single lesion in leprosy. Lepr. Rev. 67 (1996)89-94.
17. Ridley, D. S. and Jopling, W. H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966)255-273.
18. Roche, P. W., Failbus, S. S., Britton, W. J. and Cole, R. Rapid method for diagnosis of leprosy by measurements of antibodies to the M. leprae 35-kDa protein: comparison with PGL-I antibodies detected by ELISA and "dipstick" methods. Int. J. Lepr. 67(1999)279-286.
19. Sacket, D. L. Bias in analytic research. J. Chron. Dis. 32(1979)51-63.
20. Santos. A. R., Nery. J. C., Duppre. N. C., Gallo, M. E., Goes Filho, J, T., Suffys, P. N. and DeGrave, W. M. Use of the polymerase chain reaction in the diagnosis of leprosy. J. Med. Microbiol. 46(1997)170-172.
21. Scollard, D. M. Time and change: new dimensions in the immunopathologic spectrum of leprosy. Ann. Soc. Belg. Med. Trop. 73(1993)5-11.
22. Shetty, P. S., James, W. P. T. and Ferro-Luzzi, A. Malnutrition and the immune response. I. Malnutrition in the community: recent concepts. Trans. R. Soc. Trop. Med. Hyg. 88(1994)612-614.
23. Single-Lesion Multicentric Trial Group. Efficacy of single dose multidrug therapy for the treatment of single-lesion paucibacillary leprosy. Indian J. Lepr. 69(1997)121-129.
24. Stefani, M. M. A., Martelli, C. M. T, MoraisNeto, O. L., Martelli, P., Costa, M. B and Andrade, A. L. S. S. Assessment of anti-PGL-I as a prognostic marker of leprosy reaction. Int. J. Lepr. 66(1998)356-364.
25. WHO Expert Committee on Leprosy. Seventh report. Geneva: World Health Organization, 1998, p. 7, Tech. Rep. Ser. 874,
26. World Health Organization. A guide to eliminating leprosy as a public health problem. Geneva: World Health Organization, 1995, p. 21.
27. Yamamura, M., Uyemura. K., Deans, R. J., Weinberg, K., Rea, T. H., Bloom, B. R. and Modlin, R. L. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science 254(1991)277-279. 28.
28. Yoder L. J., Jacobson, R. R. and Job, C. K. A single lesion-an unusual presentation of lepromatous leprosy. Int. J. Lepr, 53(1985)554-558.
1. M.D., Ph.D, Federal University of Goias, Goiania, Goias, Brazil.
2. Ph.D., Federal University of Goias, Goiania, Goias, Brazil.
3. Ms.C., M.D.,Federal University of Rio de Janeiro, Hospital Clementino Fraga Filho, Rio de Janeiro, Rio de Janeiro, Brazil.
4. M.D., Alfredo da Matta Foundation WHO Leprosy Reference Center, Amazon, Brazil.
5. M.D., Alfredo da Matta Foundation WHO Leprosy Reference Center, Amazon, Brazil.
6. M.D., Secretariat of Health, Porto Velho, Rondonia, Brazil.
7. M.D., Federal University of Goias, Goiania, Goias, Brazil.
8. M.D., Ms.C. Federal University of Goias, Goiania, Goias, Brazil.
9. M.D., Ms.C.,Federal University of Goias, Goiania, Goias, Brazil.
10. M.D., Federal University of Goias, Goiania, Goias, Brazil.
11. M.D., Ph.D., Reference Leprosy Laboratory, Oswaldo Cruz Foundation, Rio de Janeiro, Rio de Janeiro, Brazil.
12. M.D., Ms.C., Reference Leprosy Laboratory, Oswaldo Cruz Foundation, Rio de Janeiro, Rio de Janeiro, Brazil.
13. Ph.D., Laboratory Research Branch, GWL Hansen's Disease Center at Louisiana State University, P.O. Box 25072, Baton Rouge, LA 70894, U.S.A.
14. Ph.D., Laboratory Research Branch, GWL Hansen's Disease Center at Louisiana State University, P.O. Box 25072, Baton Rouge, LA 70894, U.S.A.
15. M.D., Ph.D., Federal University of Goias, Goiania, Goias, Brazil.
Reprint requests to Dr. Celina M. T. Martelli, Universidade Federal de Goias, Instituto de Patologia Tropical e Saude Publica, Rua Delenda Rezende de Mello, S/N, Setor Universitário, Goiania/GO, Brazil 74605-050 or FAX 55-62-202-3066; email: martelli@cultura.com.br
Received for publication on 4 April 2000.
Accepted for publication in revised form on 1 August 2000.