- Volume 68 , Number 3
- Page: 277–82
Efficacy of single-dose rom therapy plus low-dose convit vaccine as an adjuvant for treatment of paucibacilfary leprosy patients with a single skin lesio
ABSTRACT
The recent World Health Organization multicentric field study on the treatment of paucibacillary (PB) leprosy patients with single skin lesion (SSL) and a single dose of rifampin-ofloxacin-minocycline (ROM) brought new hope to those who are engaged in the eradication of leprosy from India. Being encouraged by the WHO report, we undertook the present hospital-based study and found that PB leprosy patients with SSL were morphologically and histopathologically heterogeneous. The histological spectrum of SSL ranged from indeterminate through tuberculoid (TT) to borderline tuberculoid (BT) leprosy, and most patients had active BT leprosy. Ninety new, untreated PB leprosy patients with SSL were included in the present study for comparative assessment of the efficacies of ROM and ROM plus Convit vaccine therapies. Children, pregnant women, lactating mothers and patients with any thickening of nerves were excluded. All patients were bacteriologically negative (skin-smear test) but lepromin reactive. The patients were divided into two groups after proper matching for morphological and histological status of SSL: a) The test group included 60 patients and the control group included 30 patients. The test group was given a single dose of ROM initially and two injections of lowdose Convit vaccine, one initially and the other at the end of 3 months, b) The control group was given only a single dose of ROM initially. Both groups were followed clinically every 2 weeks for 6 months and retested for histological, bacteriological and lepromin status at the end of 6 months. Thereafter, they were followed clinically every month for another 6 months. In the test group, the SSL resolved in 33.3%, regressed in 48.3%, and remained active in 18.3% of the patients, while the granuloma disappeared in 70% of the cases. Only one patient developed neuritis, and in another patient the disease relapsed on the eighth month. On the other hand, the SSL in the control patients resolved, regressed and remained active in 13.3%, 63.3% and 23.3% of the cases, respectively, while the granuloma disappeared in 53.3% of the cases. In the seven patients who remained active, the disease course was progressive, and two of them developed neuritis. The clinical outcome of the patients treated with ROM plus low-dose Convit vaccine was statistically superior to those treated with single-dose ROM therapy alone.RÉSUMÉ
L'étude recente sur le terrain, organisée par l'Organisation mondiale de la Santé, concernant le traitement de la lèpre paucibacillaire (PB) avec lésion cutanée unique (LCU) par une dose unique de rifampicine associée à l'ofloxacine et al minocycline (ROM) a apporté de nouveaux espoirs chez ceux qui sont engagés dans le programme d'éradication de la lèpre en Inde. Encouragés par ce rapport de l'OMS, nous avons entrepris une étude clinique basée sur en hôpital et avons trouvé que les patients hanséniens PB avec LCU étaient morphologiquement et histopathologiquement hétérogènes. Le spectre hisologique des LCU s'étendait de lèpre indéterminée à lèpre tuberculoïde (TT) en passant par des lèpres borderline (BT), cette dernière étant la plus souvent rencontrée chez les patient, souvent d'aspect très actif. Quatre-vingt-dixneuf Patients lépreux PB non traités avec LCU furent inclus dans cette étude qui avait pour but d'évaluer et de comparer l'efficacité du traitement ROM et du traitement ROM associé au vaccin Convit. Les enfants, les femmes enceintes et les patients présentant un épaississement des nerfs furent écartés de l'étude. Tous les patients étaient négatifs à l'examen bactérioscopique mais positifs au test à le lépromine. Les patients furent divisés en deux groupes après ajustement du statut morphologique et histologique de LCU: un groupe testé (a) incluant 60 patients et un groupe témoin (b) incluant 30 patients, Il fut administré au groupe testé une seule dose de ROM initialement et deux injections de vaccin Convit à faible dose, une à l'initiation du traitement et l'ature après 3 mois. Le groupe témoin ne reçut que la dose unique de ROM initiale. Les deux groupes furent suivis cliniquement toute les 2 semaines pendant 6 mois et leur status histologique, bactériologique et au test à la lépromine fut évalué au bout de 6 mois. Après cela, ils furent suivis cliniquement tous les mois pendant une autre période de 6 mois. Dans le groupe testé, les LCU ont disparu dans 33,3% des patients, ont régressé dans 48,3% et sont restées actives dans 18,3% des patients, tandis que les granulomes ont disparu dans 70% des cas. Seulement un patient a développé une névrite et chez un autre patient, il y eut rechute de la maladie après 8 mois. En comparaison, les LCU du groupe témoin ont disparu, régressé et sont restées actives dans 13,3%, 63,3% et 23,3% des cas, respectivement, tandis que les granulomes on disparu dans 53,3% des cas. Chez les sept patients qui sont demeurés en phase active de la maladie, cette dernière a montré un caractère progressif, deux d'entre eux ayant développé des névrites. L'amélioration clinique des patients traités par ROM associé au vaccin Convit à faible dose fut statistiquement plus importante que celle des patients traités seulement par administration unique de ROM.RESUMEN
El reciente interés de la Organization Mundial de la Salud por el estúdio y tratamiento de los pacientes con lepra paucibacilar (PB) y lesiones únicas en la piei (SSL) con una sola dosis de rifampina-ofloxacinaminociclina (ROM) ha dado nuevas esperanzas a aquellos encargados de la erradicación de la lepra en la India. Estimulados por el reporte de la OMS realizamos el presente trabajo intrahospitalario y encontramos que los pacientes PB con SSL fueron morfologicamente e histopatológicamente heterogéneos. El expectro histológico de los pacientes con SSL osciló de la tuberculoide subpolar (BT). La mayoría de los pacientes tuvieron lepra BT activa. En el estúdio se incluyeron 90 casos nuevos de lepra PB con SSL para comparar la eficacia de las terapias con ROM y ROM más la vacuna de Convit. Se excluyeron dei estúdio los ninos, las mujeres embarazadas, las madres lactantes y los pacientes con cualquier engrosamiento de nervios. Todos los pacientes fueron bacteriológicamente negativos pero reactivos a la lepromina. Los pacientes se dividieron en dos grupos con características morfológicas e histológicas similares, El grupo de prueba incluyó 60 pacientes y el grupo control 30 pacientes. El grupo de prueba recibió una sola dosis de ROM al inicio y dos inyecciones de la vacuna de Convit de dosis baja, una al inicio y otra a! final del tercer mes. El grupo control recibió sólo una dosis de ROM al inicio. Ambos grupos fueron evaluados clinicamente cada dos semanas durante 6 meses y estudiados histológica, bacteriológica, e inmunológicamente (lepromina) al final dei sexto mes. Después, los pacientes fueron valorados clinicamente cada mes durante otros seis meses. En el grupo de prueba las SSL se resolvieron en 33.3% de los pacientes, regresaron en 48.3% y permanecieron activos en 18.3% de ellos, mientras que el granuloma desapareció en el 70% de los casos. Solo un paciente desarrolló neuritis y en otro paciente la enfermedad reapareció en el octavo mes. Por otro lado, las SSL en los pacientes dei grupo control se resolvieron, regresaron o permanecieron activas en el 13.3%, 63.3% y 23.3% de los cases, respectivamente, mientras que el granuloma desapareció en el 53.8% de los casos. En los siete pacientes que permanecieron activos el curso de la enfermedad de los pacientes tratados con ROM más la vacuna de Convit de dosis baja fue estadísticamente superior a la evolución de los tratados con la terapia de una sola dosis de ROM.We have just begun a new millennium, but our hope to achieve the tall objective of a world without leprosy in the near future (8) seems to be remote. It is now being realized that a poor endemic country like India, with its present socioeconomic disparity, lacking determination or political will, is far from eradicating leprosy with the current World Health Organization-recommended multidrug therapy (WHO/MDT) [Saha, K. Why India will not be able to eradicate Hansen's disease by 2000. The National Leprosy Control Program; achievements, faults and failure. Parts I and II. The Star 2 (1997) 11-15; 3 (1997) 12-15], In 1997 the Health Ministry of India reported 400,000 new leprosy cases [News and Notes. Lepr. Rev. 69 (1998) 316], Perhaps there are more hidden cases, more so in the unapproachable areas [Ganapati, R., et al. Relapse in multibacillary leprosy after rifampicin and ofloxacin treatment (Abstract) 20th Biennial Conference of the Indian Association of Leprologists, Bhopal, 1991]. It has been claimed that 50% or more of these cases are being detected at a stage when the only visible sign of the disease is a single skin lesion (SSL) (l6). A recent case-detection drive employing a modified Leprosy Elimination Campaign (MLEC) in India confirmed 454,290 new leprosy cases while examining 645 million people. Of these 454,290 cases, 53,120 (11.69%) of the patients had SSL (Report of modified leprosy elimination campaign. NLO Bull. 27 (1999) 97-103]. In an earlier survey during 1990-1995 in Gudiyatham Taluk, South India, 794 new cases of leprosy among children ranging in age from 1 to 14 years were detected. Of them, 83% had SSL (12).
It is known that lesions in 60% to 70% of paucibacillary (PB) patients regress spontaneously without any treatment (6). However, a good number of cases may eventually develop a more severe form of illness and even develop nerve damage (3).
Keeping in mind that leprosy patients with SSL might harbor only one million Mycobacterium leprae or less, a multicentric trial of treatment of PB leprosy patients with SSL with a single dose of rifampin (600 mg), ofloxacin (400 mg) and minocycline (100 mg) (ROM) was undertaken by the WHO which showed encouraging results (13). The rationale for this treatment was: a) it was operationally simple because of the economy of manpower and other resources and b) the few bacilli within monolesions would be killed by a single dose of the three bacteriocidal drugs and eliminated by the immune system of the body (4). However, leprosy is not an infectious disease like uncomplicated gonococcal gonorrhoea which, although an intracellular bacterial disease, can be successfully treated by a single megadose of penicillin. Moreover the study group did not take into account the fact that T cells play a pivotal role for killing and elimination of M. leprae (9). Further, acid-fast bacilli (AFB) (persisted) might persist intracellularly even after clinical cure with MDT although the patients could mount a Type IV hypersensitivity granulomatous reaction against M. leprae. They may multiply at any moment causing relapse, the mechanism of which is yet not fully understood (1). Thus, although SSL is morphologically a localized patch, it is a generalized disease.
Keeping in mind the relation of impairment of specific cell-mediated immunity against M. leprae with the development of leprosy, Majumder and his associates earlier treated highly bacillated Mitsuda-responsive lepromatous leprosy patients with MDT for 2 years plus six injections of lowdose Convit vaccine, which showed encouraging results (7). The present study has been undertaken to treat lepromin-responsive PB leprosy patients with SSL employing a single dose of ROM plus two injections of low-dose Convit vaccine. The results of the chemo-immunotherapy have been compared with the results of treatment of similar patients with a single dose of ROM therapy only. The rationale for adding low-dose Convit vaccine to ROM therapy was to confer on the macrophages the capacity to quickly clear intracellular M. leprae after they were killed by ROM therapy.
MATERIALS AND METHODS
Ninety, fresh, untreated leprosy patients (18 males and 72 females) were selected for the trial from the Out-Patients' Department of the School of Tropical Medicine, Calcutta, India. Their age range was from 15 to 60 years. Children below the age of 15 years and pregnant and lactating women were excluded from the study. The study extended from January 1998 to November 1999. The diagnosis of leprosy was based on thorough clinical examinations, bacteriological (slit-skin smear test) and histological findings as well as lepromin testing. All patients were Mitsuda reactive and smear negative. Based on the clinical features, SSL patients were classified into eight types (Table 1). Patients with palpable nerves were excluded from the study. SSL patients were tested for thermal, touch and pain sensations which were impaired in all patients. Histological staging of SSL cases was based upon the criteria of Ridley and Jopling. There were 10 indeterminate, 9 TT and 71 BT leprosy patients. Lepromin testing was performed employing standard WHO armadillo-derived lepromin. Late lepromin responsiveness was recorded and graded as described by Jopling and McDougall (5). Six patients showed 1+, 20 showed 2+ and 64 showed 3+ positivity.
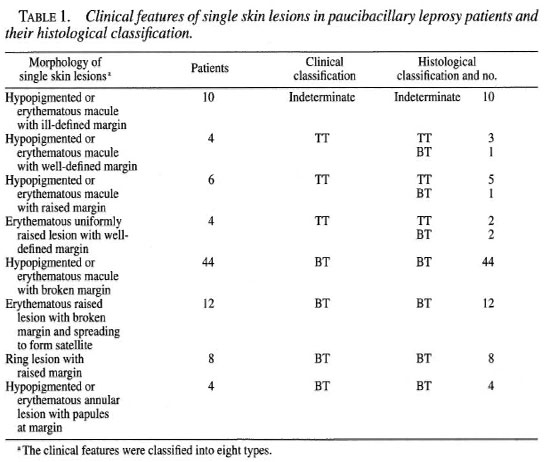
Location of SSL
The majority of monolesions were found on the uncovered parts of the body, being detected by the patients themselves (Table 2).
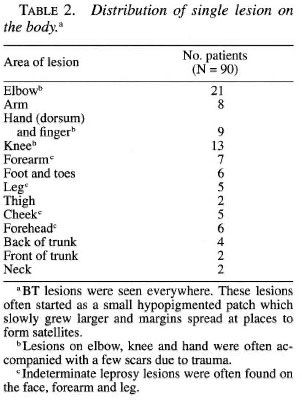
Grouping of patients
Ninety patients were divided into two groups: a) Test group included 60 patients and b) control group included 30 patients (Table 3). The grouping was done after matching the types of clinical features and histological types of SSL as far as possible
ROM therapy
Rifampin (600 mg), ofloxacin (400 mg) and minocycline (100 mg) were given once to all patients.
Vaccine
Low-dose Convit vaccine (7) contained 1.6 x 107 heat-killed M. leprae (A) in 0.1 ml saline and 1.5 x 107 BCG (Japan) in 0.1 ml saline.
Treatment administered
At the start of treatment, patients in both groups were each given a single dose of ROM; in addition, the test group was administered two injections of low-dose Convit vaccine, one initially and another after 3 months. All patients were followed up clinically every 2 weeks from the beginning of treatment for 6 months and thereafter every month for another 6 months. At the end of the first 6 months bacteriological, histological and immunological (lepromin test) assessments were repeated.
RESULTS
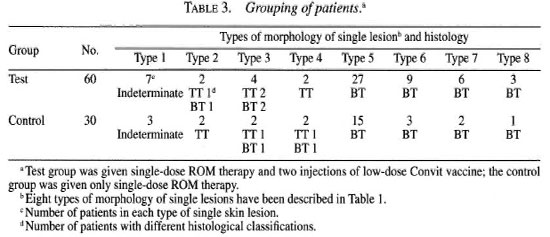
Out of 90 patients with SSL, 71 (82%), 9 (6.6%) and 10 (11.4%) patients had BT, TT and indeterminate leprosy, respectively. The size of the lesions varied from ½ to 2 inches (approximately 1.3 to 5.1 cm) in diameter. There were 48 BT, 5 TT and 7 indeterminate leprosy patients in the test group and 23 BT, 4 TT and 3 indeterminate leprosy patients in the control group. The clinical features of SSL in our leprosy patients showed macular, raised or ring lesions, 64 of which were active and tended to spread and form satellites. This indicated that our hospital-based study included an advanced form of SSL.
Criteria of assessment of clinical and histological outcome following treatment
Clinical outcome. The clinical outcome was of three types: a) resolution and healing with or without scar formation; b) regression or quiescent (decrease in size, hypopigmentation or erythema became faint, no visible margin); c) active or treatment failure (faint hypopigmentation or erythema with visible margin and no decrease in size and infiltration) (Table 4). Resolution and regression were taken as clinical benefits. Scarring was due to collagen damage following immunological insult and dermal reaction (15).
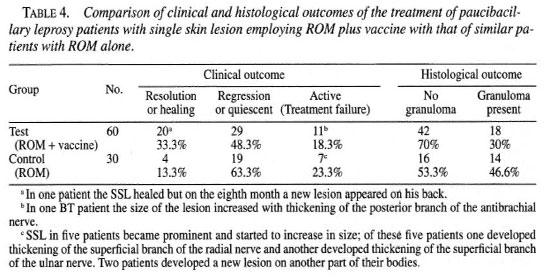
Histological outcome. The histological outcome was of two types: a) no granuloma and b) granuloma persisting (Table 4). No granuloma or histological resolution was taken as a histological benefit. It is a silent process which does not leave apparent fibrosis (16). The presence of granuloma shows a preponderance of young macrophages with few vacules (l5).
There was no adverse drug reaction during the course of treatment and follow-up period. Complete return of sensations was observed in 24 patients showing clinical resolution; partial improvement of sensations was seen in 48 cases showing quiescent lesions and no improvement of sensations was observed in 18 active or treatment-failure cases (Table 4).
Comparison of clinical, histological and immunological outcome in patients receiving ROM plus vaccine or ROM
Clinical benefit. In the test group, 49 out of the total of 60 patients (81.6%) (20 resolved; 29 regressed) showed clinical benefit; whereas in the control group 23 out of the total of 30 patients (76.6%) (4 resolved; 9 regressed) showed clinical benefit (Table 4). The difference was statistically significant (χ2 = 6.62; 0.02> p >0.01).
Resolved skin lesions in the test and control groups were 33.3% and 13.3%, respectively (Table 4). The difference was statistically significant (χ2 6.70; 0.01 > p >0.001).
Scarring of lesion in the test group was 28% and 13.3% in the control group (Table 4). The difference was not statistically significant (χ2 = 1.99; 0.2> p >0.1).
There were 11 treatment failures (18.3%) out of the 60 cases in the test group and 7 (23.3%) out of 30 cases of the control group (Table 4). Among the 11 treatment-failure cases in the test group one BT patient presented features of upgrading reaction in the skin lesion and in the posterior antibrachial cutaneous nerve. Another patient suffered a relapse after 8 months (Table 4).
All seven treatment-failure cases in the control group downgraded. Two cases showed neuritis of the superficial radial cutaneous and superficial branch of the ulnar nerve (Table 4). The percentage of complications, such as clinical worsening of the disease, neuritis and relapse, was significantly less in the test group than in the control group (χ2 = 10.38; 0.1> p >0.05).
Histological benefit. In the test group the histological benefit (disappearance of granuloma) was observed in 42 (70%) out of the 60 patients while in the control group granuloma disappeared in 16 (53.3%) of the 30 cases. The difference was not statistically significant (χ2 = 3.46; 0.1 > p > 0.05).
Immunological benefit. Before starting treatment the degree of lepromin positivity was 1+ in 6 patients, 2+ in 20 patients and 3+ in 64 patients.
In the test group one BT patient with an initial lepromin positivity of 1+ upgraded to 2+, and two other BT patients with an initial 2+ positivity upgraded to 3+ following the administration of ROM plus vaccine. In the remaining patients the lepromin reactivity showed no change. In the control group there was no change in the lepromin status following ROM therapy.
DISCUSSION
Single skin lesion (SSL) and problems of its diagnosis
In a field study like that conducted by the WHO, an accurate diagnosis of SSL, especially in females belonging to conservative Indian society, was difficult, if not impossible. Often a patient with SSL in the exposed part might have one or more lesions in unexposed areas. Since our study was hospital based, we could very cautiously confirm our diagnosis. Furthermore, a single skin lesion is morphologically as well as histologically heterogeneous. SSL sizes in our patients varied from ½ to 2 inches and clinical features were variable (Table 1). There were 64 active SSL, which tended to spread. The histological spectrum of SSL in our patients extended from indeterminate through TT to BT leprosy, and BT leprosy patients were in the majority (82%). On the other hand, in the WHO field survey more cases of indeterminate and TT leprosy might have been found and included in their study(3). In the WHO study patients with nerve trunk involvement were excluded. A recent histological study had shown that even indeterminate leprosy patients might have associated involvement of deep dermal nerves. We took special care to exclude all patients with any suspected thickening of any nerve. It must be emphasized that all of the factors mentioned above might influence the clinical and histological outcome of patients with SSL after ROM therapy.
Clinico-histological outcome of ROM plus vaccine and ROM therapies
The leprosy laboratory manual of the WHO classifies activity of disease into two, i.e., active and quiescent or in regression(15). The criteria for the assessment of the clinical outcome in our patients after treatment were of three types: resolved, regressed and active (Table 4). The histological activity is the basis of clinical course of the disease, i.e., progressive or resolved (15). We had two classifications for histological outcome: a) no granuloma seen (clinically resolved or healed) and b) granuloma present (clinically active or treatment failure and some quiescent cases) (Table 4). Eighteen BT cases (11 out of the 60 of the test group patients and 7 out of the 30 control group patients) remained clinically and histologically active after treatment. Of them one in the test group and two in the control group developed neuritis, perhaps indicating reversal reaction. However, none in the quiescent group developed any complication during the study period. Unexpectedly, one BT leprosy patient belonging to the test group showed resolution of SSL, but developed a new lesion on his back in the eighth month, indicating relapse (Table 4). There was no relapse in the control group.
Why 18% to 20% of patients with SSL remained active after ROM plus vaccine or ROM therapy, respectively
Inadequate clinical response in PB leprosy patients following standard MDT despite being Mitsuda responsive has been reported (2). A few BT leprosy patients might harbor AFB (persisters) in their nerves even though they became clinically inactive following MDT therapy-an unfortunate fact repeatedly overlooked by many field workers. These persisters might evade the macrophage-mediated killing of M. leprae, causing reversal reaction and relapse even in lepromin-reactive patients years after release from treatment. Persisters possess highly resistant outer coats and surround themselves with phenolic glycolipid, which scavenges free radicals. They also release a lipoarabinomannan which blocks the ability of macrophages to respond to interferon gamma (IFN-γ). Moreover, the infected macrophages may lose their efficiency as antigen-presenting cells (11). These factors might be the cause of so many patients remaining active after ROM plus vaccine and ROM therapies (Table 4). Thus, single-dose ROM therapy, although operationally simple and economically advantageous, has its own inherent shortcomings, namely, persisters may not be killed and may remain alive in macrophages which might cause reaction and relapse.
How to eliminate persisters; rationale of ROM plus vaccine therapy
In a recent study, Chaudhuri, et al. (2) pointed out that it was not Mitsuda positivity but the bacteria-clearing capacity of the macrophage that was the real indicator of protective immunity against M. leprae. This important macrophage function depends on the helper T-cell function and genetically competent macrophage (2-9). ROM can kill most M. leprae but not persisters. Neither can it improve the bacteria-clearing capacity of macrophages nor can it boost the Tcell function. On the other hand, an injection of Convit vaccine containing M. leprae plus BCG produces IFN-y, which helps human macrophages to express an I-hydrolase enzyme which converts the circulating inactive 25-hydroxycholecalciferol (vitamin D3) into active metabolite, 1,25-cholecalciferol (calciferol). This metabolite activates the antimycobacterial mechanism of the macrophages, leading to the killing of M. leprae (11). This notion supports our data that there were fewer complications (2.3%) in our test group than in our control group (23.3%). In the test group one patient relapsed and another developed neuritis; in the control group two patients developed neuritis and in five patients the disease went on a downhill course (Table 4). Why one patient, although receiving ROM plus the vaccine, relapsed on the eighth month after SSL resolved is not known. Perhaps the vaccine cannot correct genetically determined macrophages, which are susceptible to M. leprae infection (9).
Comparison of WHO study with present study
The WHO study was field-based, while the present study was hospital-based. The WHO study was on clinical assessments only, while our patients were assessed both clinically and histologically and immunologically. Only 0.9% of the patients receiving ROM in the WHO study showed treatment failure, while 23.3% of the patients receiving ROM in our present study remained clinically active, indicating treatment failure. The occurrence of reaction and neuritis was less in the WHO study than in the present study; thus, only 1.3% of the patients receiving ROM in the WHO study developed reaction while 6.6% patients receiving ROM in the present study developed neuritis and had mild reactions (Table 4). The higher incidence of treatment-failure cases and the higher occurrence of reversal reactions in the present study could be attributed to the inclusion of a large number of active BT leprosy patients. We do not know the histological status of the SSL in the patients of the WHO study.
We conclude that SSL is not a single entity but is morphologically and histopathologically heterogeneous. There is significant clinical benefit in the treatment of paucibacillary leprosy patients with SSL by single-dose ROM therapy plus two injections of low-dose Convit vaccine as compared to the treatment of similar patients with ROM therapy alone. An increase in the dosage and the number of vaccine injections may yield better results.
REFERENCES
1. Britton, W. J. The management of leprosy reversai reactions. Lepr. Rev. 69(1998)225-239.
2. Chaudhuri, S., Hajra, S. K., Mukerjee, A., Saha, B., Majumder, B., Chattapadhya, D. and Saha, K. Why relapse occurs in PB leprosy patients after adequate MDT despite they are Mitsuda reactive; lessons from Convit's experiment on bacterial clearing capacity of lepromin-induced granuloma. Int. J. Lepr. 66(1998)82-89.
3. Ekambaram, V. and Sithambaran, M. Self healing in non-lepromatous leprosy in the area of ELEP Leprosy Control Project, Dharampuri(Tamilnadu). Indian J. Lepr. 49(1977)387-392.
4. Grosset, J. Wither short course chemotherapy in leprosy.(Editorial)Indian J. Lepr, 69(1997) 119-120.
5. jopling, w. H. and McDougall, a. C. Handbook of Leprosy. 5th edn. New Delhi: C.B.S. Publisher and Distributors, pp. 54-57.
6. Lara, C. B. and Nolase, J. O. Self healing or abortive and residual form of childhood leprosy and their significance. Int. J. Lepr. 24(1956)246-263.
7. Majumder, V., Mukerjee, A., Hajra, S. K., Saha, B. and Saha, K. Immunotherapy of far-advanced lepromatous leprosy patients with lowdose Convit vaccine along with multidrug therapy(Calcutta trial). Int. J. Lepr. 64(1996)26-36.
8. Noordeen, S. K. Eliminating leprosy as a public health problem; why the optimism is justified. Int. J. Lepr. 63(1995)559-566.
9. Ottenhoff, T. H. M. Immunology of leprosy: lessons from and for leprosy. Int. J. Lepr. 62(1999)108-121.
10. Rajdekar, M. P., Thaker, U. H., Pharande, A. M,, Naik, S. S. and Ganapati, R. Hidden sources of new infection in unapproachable areas. Indian J. Lepr. 69(1997)169-175.
11. Roitt, I., Brostoff, J. and Male, D. Immunology. 5th edn. London: Mosley, 1998, pp. 235-238.
12. Selvasekar, A., Geetha, J., Nisha, K., Manimozhi, N., Jesudasan, K. and Rao, P. S. S. Childhood leprosy in an endemic area. Lepr. Rev. 70(1999)21-27.
13. Single Lesion Multicentric Trial Group. Efficacy of single dose multidrug therapy for the treatment of single lesion paucibacillary leprosy. Indian J. Lepr. 69(1997)121-129.
14. Suneetha, S., Arunthathi, S., Chandi, S., Kurian, N. and Chacko, C. J. G. Histological studies in primary neuritic leprosy: changes in the apparently normal skin. Lepr. Rev. 69(1998)351-357.
15. World Health Organization. Laboratory techniques for leprosy. Geneva: World Health Organization, 1986, pp. 38-44.
16. World Health Organization. Progress towards the elimination of leprosy as a public health problem. Wkly. Epidemiol. Rev. 71(1996)149-156.
1. M.B.B.S., Medical Officer; Department of Leprology,School of Tropical Medicine, Calcutta, India.
2. M.D., D.T.M.&H., Assistant Professor; Department of Leprology,School of Tropical Medicine, Calcutta, India.
3. M.B.B.S., D.C.P., Ph.D., Reader (Retired); Department of Leprology,School of Tropical Medicine, Calcutta, India.
4. M.D., Lecturer, Department of Leprology,School of Tropical Medicine, Calcutta, India.
5. M.Sc., M.B.B.S., Ph.D. (U.S.A.), Professor of Immunology (Retired), Delhi University, Delhi, India.
Reprint requests to Kunal Saha, Ph.D., 45-A SovaBazar Street, Calcutta 700 005, India.
Received for publication on 24 January 2000.
Accepted for publication in revised form on 12 July 2000.