- Volume 68 , Number 3
- Page: 291–8
Sensory testing with monofilaments in hansen's disease and normal control subjects
ABSTRACT
Sensory testing with Semmes-Weinstein filaments was conducted on: 112 normal subjects to determine the effects of age, gender and occupation on threshold perception, 27 Hansen's disease (HD) patients to determine inter-observer and intra-observer reliability of testing, and 101 patients with HD and a history of hand and/or foot ulceration to identify thresholds for injury risk. Filament thresholds were found related to age (p <0.002) and occupation (p <0.001) but not gender (p >0.1). Inter-observer and intra-observer reliability was found to be high (intraclass correlation coefficient = 0.88-0.93). The 4.93 (7.0-7.7 g) filament had 97% sensitivity and 100% specificity for identifying a history of foot injuries, and the 4.17(1.2-1.6 g) filament had 100% sensitivity and 100% specificity for identifying hand injuries.RÉSUMÉ
Des tests sensoriels utilisant les filaments de Semmes-Weinstein furent utilisés pour tester: 112 subjets normaux afin de déterminer l'influence de l'âge, le genre et la profession sur les limites de perception sensorielle, 27 patients hansèniens (HD) afin de déterminer la répétitivité au sein d'un même et entre les manipulateurs et 101 patients hansèniens présentant des commémoratifs d'ulcères aux pieds et/ou aux mains pour identifier des limites de perception sensorielle associées à de forts risques de blessures. Une relation entre les limites de perception sensorielle et l'âge (p <0,002), la profession (p <0,001), mais pas le genre (p >0,1) fut établie: La reproductibilité inter et intra-utilisateur fut trouvée être forte (coefficient de corrélation intra-classe = 0,88-0,93). Le filament numéro 4.93 (7,0 à 7,7 g) a présenté 97% de sensibilité et 100% de spécificité pour identifier des patients avec des commémoratifs de blessures aux pieds et le filament numéro 4.17 (1,2 à 1,6 g) a présenté 100% de sensibilité et 100% de spécificité pour identifier des patients avec des commémoratifs de blessures aux mains.RESUMEN
Se practicaron pruebas sensorials con los filamentos de Semmes-Weinstein en: (a) 112 individuos normales para determinar los efectos de la edad, género y ocupaciôn en el umbral de perception, (b) 27 pacientes con la enfermedad de Hansen (HD) para determinar la confiabilidad dentro y entre los ejecutores de la prueba, y (c) 101 pacientes con HD y una historia de ulceraciön en la mano o el pie para identificar los umbrales de riesgo de dano. Los umbrales de sensibilidad a los filamentos estuvieron relacionados con la edad (p <0.002) y ocupaciôn (p <0.001) pero no con el género (p >0.1). La confiabilidad dentro y entre los ejecutores de la prueba fue alta (coeficiente de corrclaciön intraclase: -0.880.93). Los filamentos 4.93 (7.0-7.7 g) tuvieron una sensibilidad del 97% y una especificidad del 100% en la identification de una historia de lesiones en el pie, y el filamento 4.17 (1.2-1.6 g) tuvo una sensibilidad del 100% y una especificidad del 100% en la identification de lesiones en la mano.Monofilaments have been widely used in Hansen's disease (HD) to monitor sensory loss in hands and feet, provide a guide for the treatment of reactions and identify patients at risk of injury (4,8,11,16, 24, 27, 35). There are limited and inconclusive data on normal threshold values (6,7,17,31,36,37) and limited statistical evidence for the reliability of testing with nylon filaments (5,10, 21, 22, 26).
The use of a standardized set of nylon filaments for sensory testing was first reported by Semmes, Weinstein, Ghent and Teuber in 1960 (31). Their original 20 filament set was calibrated to incrementally bend at forces that were approximately log linear. Filaments were identified by their corresponding marking numbers derived by the formula log10 [10(bending force mg)]. Semmes-Weinstein filaments (SWF) are commercially available and are designed after the filament dimensions (diameter and length) of the original set (31).
Reported SWF thresholds for the hand of normal subjects vary between 2.83 (6,7,36) and 3.61 marking numbers (17, 23), and for the feet of normal subjects between 3.61 (7) and 4.31 marking numbers (17,23). Sensibility has been shown to be related to age, gender and anatomic location (2,10,19,31,36). Sensory thresholds have been found to be lower in females than males, lower in the face than the hands, lower in the hands than the feet and lower in younger subjects. A basis for reported gender differences has not been found. The density of sensory end organs, such as Meissner's corpuscles, has been shown to vary by anatomic location and age (12). Much of previous SWF normative data, however, has been based on young adult populations.
The reproducibility of SWF testing has been reported to be good when filament length and diameter are standardized (5). High inter-observer and intra-observer reliability has been found when testing normal subjects (31), and testing the hands, feet and eyes of HD patients and the feet of diabetes patients using mini-sets (3-4 filaments) of nylon filaments (3, 21, 22, 26, 29, 34). Inter-observer reliability has been shown to be dependent upon the training and experience of the observers (26). The reliability of testing patients using the full set of 20 SWF has not been reported.
Loss of protective sensation predisposes an individual to mechanical, thermal or chemical injury (8). Threshold levels predictive of loss of protective sensation have been proposed for the hand (6, 18, 19, 36) and the foot (9, 13-15, 20, 24, 25, 32, 33). There is no agreement on the level of protective sensation for either the hand or the foot. The use of the 10-g monofilament has been shown to be predictive of risk for foot problems in diabetes (30), and has been adopted as a standard of care by the American Diabetes Association (1).
This study was designed to: determine the effects of age, gender and occupation on SWF thresholds in normal subjects, test the inter-observer and intra-observer reliability of the full SWF set on HD patients and identify thresholds for hand and foot injuries in an HD population.
MATERIALS AND METHODS
Instruments. Sensory testing was performed using two sets of SWF (North Coast Medical Inc., San Jose, California, U.S.A.), each consisting of 20 ordered instruments. The bending forces of the filaments used in this study were measured using a strain gauge recorded off an oscilloscope as previously described (5) and compared with the theoretical design (31) (Table 1). The measured bending forces generally follow a log linear pattern similar to the theoretical design reported by Semmes and Weinstein (Fig. 1).
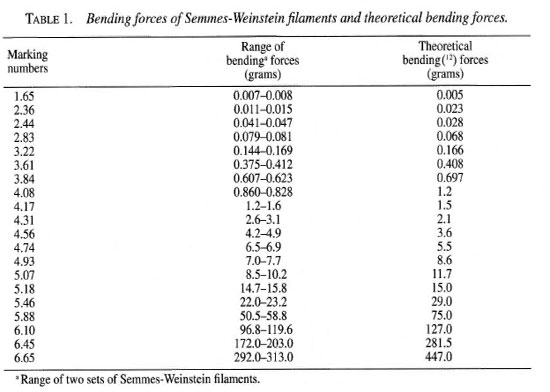
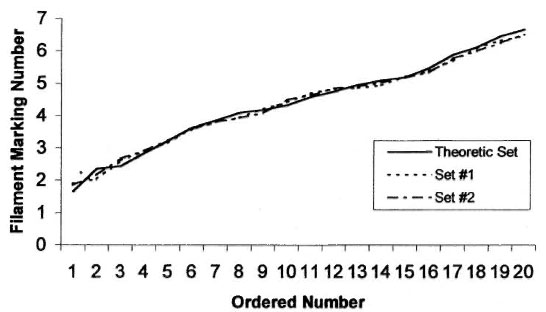
Fig. 1. Log linear distribution of two commercial Semmes-Weinstein filament sets compared to the theoretical set.
Filaments were applied perpendicular to the skin of subjects for a duration of 1-2 seconds. Three touches were made randomly to each area tested on the hand and foot. Filaments were applied in an ascending order. The 6.65 marking number filament was not used in testing hands because it was felt to be too stiff for use on the finger or palm. The threshold was determined by a subject's verbal response to at least two out of three touches (9). This method provides less chance of guessing than a one out of three (6) and is less time consuming than averaging the mean of thresholds (31)Testing was performed in an isolated room to control noise. Subjects were seated with the hands and feet comfortably supported during testing. A curtain shielded subjects from view of filament touches. Testing was performed on HD patients, workers and visitors at the McKean Rehabilitation Center, Chiang Mai, Thailand.
Thresholds in normal control subjects. Sensory testing was performed on 53 male and 59 female Thai subjects who had no history of HD or other neuropathic conditions. Subjects were a shoe-wearing population. The mean age of normal subjects was 39.5 ± 12.7 (range 18-66 years). Filament tests were performed on the nondominant side of the body at the volar distal phalanx of the fifth finger, hypothenar eminence of the palm, plantar distal phalanx of the great toe, medial arch of the foot, and plantar heel of the foot. The nondominant side of the body was tested to reduce the likelihood of nerve damage associated from overuse syndromes. Occupation was rated in 64 subjects as: heavy, moderate and light work based on interview and income status. The heavy work group were primarily farmers and laborers, and the light work group were mainly medical, clerical and professional workers. Occupations that were not primarily sedentary or manual labor were rated as moderate work. These occupation groups were used to determine if physical labor was associated with reduced hand and foot sensibility.
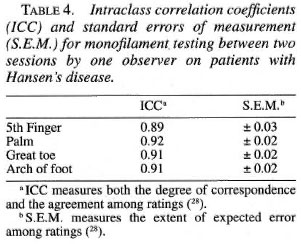
Reliability of SWF testing. Testing was performed on 25 hands and 25 feet of 27 HD patients with sensory neuropathy. Patients who could not perceive any of the filaments were eliminated from the study. Two experienced therapists tested the predetermined anatomic locations on the fifth finger, palm, great toe, and arch of the foot at one session, and one observer tested the same locations at a second session the following day.
Thresholds on areas of ulceration. Thresholds were determined in 101 HD patients, 72 with a history of hand ulcerations and 68 with a history of foot ulcerations. Determination of neuropathic ulceration was based on inspection and a history of chronic or repeated wounds. Raters were careful to perform testing outside the margin of a wound scar or callus formation.
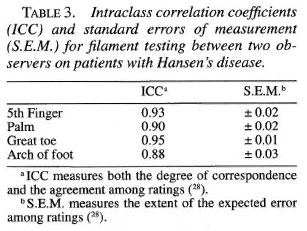
Analysis. Regression analysis was used determine the relationship between age, gender, and occupation on sensory thresholds in normal subjects. A multiple range test at the 0.05 alpha level was used for comparisons among age, gender and occupation groups. Filament thresholds were reported in marking numbers. Filament marking numbers are log transformations of filament bending forces which are more suitable for statistical analysis. Intraclass correlation (ICC) and standard error of measurement (SEM) was used to determine the inter-observer and intra-observer reliability of filament measurements. ICC is a reliability index that measures both the degree of correspondence and the agreement among ratings, and the SEM measures the expected error among ratings (28).
RESULTS
Analysis showed sensory threshold was related to age (p <0.002). Filament thresholds were lower in the 18-29 age group compared to the 30-49 age group and lower in the 30-9 year age group compared to the 50-69 year age group (Table 2). There was no relationship between filament thresholds and gender (p >0.01) (Fig. 2).
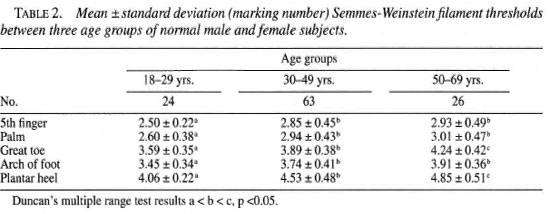
Fig. 2. Mean hánd and foot Semmes-Weinsteinfilament thresholds for male and female groups of normal subjects.
There was a difference in sensory thresholds among occupational levels (p <0.001). Light labor occupations had lower filament thresholds, in both the hands and the feet, than heavy and medium labor occupations (Fig. 3).
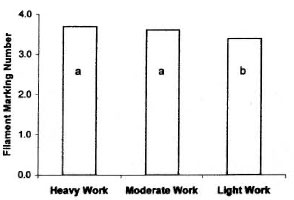
Fig. 3. Mean hand and foot Semmes-Weinsteinfilament thresholds for three work groups of normalmale and female subjects. Duncan's multiple range testresults a> b, p <0.05.
Intraclass correlations were high and standard errors of measurement were low for both inter-observer (Table 3) and intraobserver (Table 4) testing at the sites tested.
There was a clear discrimination between the frequency distributions of sensory thresholds in HD patients with a history of ulceration and normal controls for both the foot (Fig. 4) and the hand (Fig. 5). The 4.93 (7.0-7.1 g) SWF had 97% sensitivity and 100% specificity for discriminating patients with ulcerations of the foot. The 4.17 (2.6-3.1 g) SWF had 100% sensitivity and 100% specificity for discriminating patients with ulcerations of the hand.
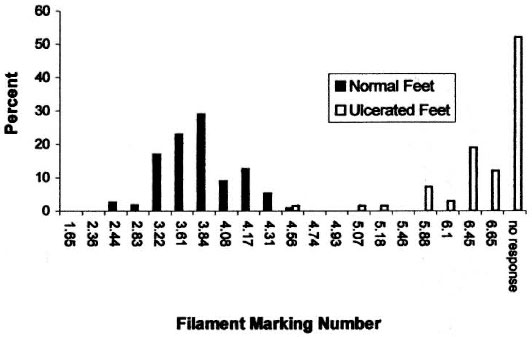
Fig. 4. The frequency distribution of Semmes-Weinstein filament thre sholds in normal subjects and in Hansen's disease patients with a history of foot ulceration.
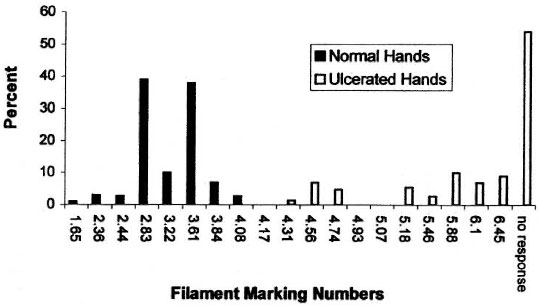
Fig. 5. The frequency distribution of Semmes-Weinstein filament thresholds in normal sujects and in Hansen's disease patients with a history of hand ulceration. The 6.65 marking number filament was not used intesting hands because it was felt to be too stiff for use on the finger or palm.
DISCUSSION
This study finds good reliability when testing HD patients using the full set of 20 SWF by experienced observers, and agrees with previous studies that were performed using filament mini-sets on patients (3, 21, 22, 26, 29, 34) and the full SWF set on healthy subjects (31). In this study the estimated normal thresholds would be the 3.84 (marking number) filament for the hand and the 4.31 filament for the foot (Table 3). These data generally agree with the upper limits of previously published normal values for the hand (3.61 marking number) (17, 23) and the foot (4.31 marking number) (17, 23). These and comparative normal values have been based on an approximate 95% confidence interval where 97 of 100 normal individuals tested would be within the normal threshold range. Lack of agreement among published normal data could be explained by a measurement error resulting from differences in instrument design and wear, criteria for threshold determination, environmental conditions, differences in age, occupation or skin callus.
In this study, age and occupation were significantly related to SWF thresholds. In the absence of age adjusted norms, clinicians should be cautious not to overstate sensory loss in older individuals. This study found that subjects in heavy or mediumheavy occupations had higher sensory thresholds than those engaging in light occupations. While this study did not assess the amount of walking or hand activity on the job, the authors speculate that individuals performing heavy work are likely to develop more callus on the hands and feet, which could increase the threshold of sensory perception. The effect of callus may be quite significant in the feet of subjects who often walk barefoot. Normal data should be developed specifically for a population who usually walk barefoot. This study did not find SWF thresholds to be lower in females as reported in previous studies. The gender effect reported in previous studies may be related to occupation and the higher presence of callus in males.
In this study the 4.93 (7.0-7.1 g) filament in the foot and the 4.17 (1.2-1.6 g) filament in the hand had optimal sensitivity and specificity for discriminating between normal individuals and those with a history of hand and foot ulceration. These threshold levels are similar to previous reported threshold levels for individuals with a history of hand (19, 24) and foot ulcerations (9,14, 15). Future prospective research is needed to test the accuracy of these threshold levels in predicting risk for ulceration.
Acknowledgment. This study was supported by a grant from the Gastmann-Wichers Foundation in The Netherlands.
REFERENCES
1. American Diabetes Association. Foot care in patients with diabetes mellitus. Diabetes Care 21(1998)S54.
2. Anderson, A. m. and van Brakel, W. H. Agespecific normal thresholds for sensibility testing with monofilaments in a Nepali population.(Abstract)Int. J. Lepr. 66(1994)69A.
3. Anderson, A. M. and Croft, R. P. Reliability of Semmes-Weinstein monofilament and ballpoint sensory testing and voluntary muscle testing in Bangladesh. Lepr. Rev. 70(1999)305-313.
4. Bell-Krotoski, J. A study of peripheral nerve involvement underlying physical disability of the hand in Hansen's disease. J. Hand Ther. 5(1992)133-141.
5. Bell-Krotoski, J. and Tomancik, E. The repeatability of testing with Semmes-Weinstein monofilaments. J. Hand Surg. 12(1987)155-161.
6. Bell-Krotoski, J. A. Light touch-deep pressure testing using the Semmes-Weinstein monofilaments. In: Rehabilitation of the Hand. 3rd edn. Hunter, J., Schneider, L., Mackin, E. and Callan, A., eds. St. Louis: C.V. Mosby Co., 1990, pp. 585-593.
7. Bell-Krotoski, J. A., Fess, E. E. Figarola, J. H. and Hiltz, D. Threshold detection and SemmesWeinstein monofilament. J. Hand Ther. 8(1995)155-162.
8. Birke, J. A., Patout, C. A. and Foto, J. G. Factors associated with ulceration and amputation in the neuropathic foot. J. Orthop. Sports Phys. Ther. 30(2000)91-97.
9. Birke, J. A. and Simms, D. S. Plantar sensory thresholds in the ulcerative foot. Lepr. Rev. 57(1986)261-267.
10. Bloom, S., Till, S., Sonksem, P. and Smith, S. Use of a biothesiometer to measure individual vibration thresholds and their variation in 519 nondiabetic subjects. Br. Med. J. 288(1984)233-235.
11. Cochrane, R. G., McRobert, G. and Davey, T. F. Leprosy in Theory and Practice. 2nd edn. Baltimore: The Williams and Wilkins Co., 1964, pp. 209-212.
12. Dyck, P. J., Darenes, J., O'Brien, P. C. and Zimmerman, I. R. Detection thresholds of cutaneous sensation in humans. In: Peripheral Neuropathy. 3rd edn. Dyck, P. J., Thomas, P. K., Lamber, E. H. and Bunge, R., eds. Philadelphia: W. B. Saunders, 1993, pp. 706-728.
13. Dorairaj, A., Reddy, R. and Jesudasan, K. An evaluation of the Semmes-Weinstein 6.10 monofilament as compared with 6 nylon in leprosy patients. Indian J. Lepr. 60(1988)413-417.
14. Hammond, C. J. and Klenerman, P. Protective sensation in the foot in leprosy. Lepr, Rev. 59(1988)347-354.
15. Holewski, J. J., Stess, R. M., Graf, P. M., et al. Aesthesiometry: quantification of cutaneous pressure sensation in diabetic peripheral neuropathy. J. Rehabil. Res. Dev. 25(1988)1-10.
16. Jamison, D. Sensitivity testing as a means of differentiating the various forms of leprosy found in Nigeria. Int. J. Lepr. 39(1971)504-507.
17. Kets, C. M., Van Leerdam, M. E., van Brakel, W. H., Deville, W. and Bertelsmann, F. W. Reference values for touch sensibility for thresholds in healthy Nepali volunteers. Lepr. Rev. 67(1996)28-38.
18. Kuipers, M., Owen, B. M. and Stratford, C. J. Assessment of the methods available for testing sensation in leprosy patients in a rural setting. Lepr. Rev. 66(1995)55-62.
19. Kuipers, M. and Schreuders, T. The predictive value of sensation testing in the development of neuropathic ulceration on the hands of leprosy patients. Lepr. Rev. 65(1994)253-261.
20. Kumar, S., Fernando, D. J. S., Veves, A., etal. Semmes-Weinstein monofilaments: a simple, effective and inexpensive screening device for identifying diabetic patients at risk of foot ulceration. Diabetes Res. Clin. Pract. 13(1991)63-68.
21. Lewis, S. Reproducibility of sensory testing and voluntary muscle testing in evaluating the treatment of acute neuritis in leprosy patients. Lepr. Rev. 54(1983)23-30.
22. Lienhardt, C., Currie, H. and Wheeler, J. G. Inter-observer variability in the assessment of nerve function in leprosy patients in Ethiopia. Int. J. Lepr. 63(1995)62-76.
23. Malaviya, G. N., Husain, S., Girdhar, A. and Girdhar, B. K. Sensory functions in limbs of normal persons and leprosy patients with peripheral trunk damage. Indian J. Lepr. 66(1994) 157-164.
24. Malaviya, G. N., Husain, S., Mishra, B., Girdhar, A. and Girdhar, B. K. Protective sensibility -its monofilament nylon threshold equivalents in leprosy patients. Indian J. Lepr. 69(1997)149158.
25. McGill, M., Molyneaux, L., Spencer, R., Heng, L. F. and Yue, D. K. Possible sources of discrepancies in the use of the Semmes-Weinstein monofilament. Diabetes Care 22(1999)598-602.
26. Mueller, M. J., Diamond, J. E., Delitto, A. and Sinacore, D. R. Insensitivity, limited joint mobility and plantar ulcers in patients with diabetes mellitus. Phys. Ther. 69(1989)453-159.
27. Naafs, B. and Dagne, T. Sensory testing: a sensitive method in the follow-up of nerve involvement. Int. J. Lepr. 45(1978)364-368.
28. Portney, L. G. and Watkins, M. P. Foundations of Clinical Research: Applications to Practice. Norwalk, Connecticut: Appleton & Lange, 1993, p, 509.
29. Premkumar, R., Daniel, E., Suneetha, S. and Yovan, P. Quantitative assessment of facial sensation in leprosy. Int. J. Lepr. 66(1998)348-354.
30. Rith-Najarian, S. J., Stolusky, T. and Gohdes, D. M. Identifying diabetic patients at high risk for lower-extremity amputation in a primary health care setting: a prospective evaluation of simple screening criteria. Diabetes Care 15(1992)1386-1389.
31. Semmes, J., Weinstedm, S., Ghent, L. and Teuber, H. Somatosensory Changes after Penetrating Brain Wounds in Man. Cambridge: Harvard University Press, 1960, pp. 4-11, 59-62.
32. Sonesko, J. M., Kato, M., Soto, R. and Bild, D. E. Comparison of quantitative sensory threshold measures for their association with foot ulceration in diabetic patients. Diabetes Care 13(1990)1057-1061.
33. Stratford, C. J. and Owen, B. M. The effect of footwear on sensory testing in leprosy. Lepr. Rev. 65(1994)58-65.
34. van Brakel, W, H., Khawas, I. B,, Burung, K. S., Kets, C. M, van Leerdam, M. E. and Drever, W. Intraand inter-tester reliability of sensibility testing in leprosy. Int. J. Lepr. 64(1996)287-298.
35. van Brakel, W. H., Shute, J., Dixon, J. A. and Arzet, H. Evaluation of sensibility in leprosy: comparison of various clinical methods. Lepr. Rev. 65(1994)106-121.
36. Von Prince, K. and Butler, B. Measuring sensory function of the hand in peripheral nerve injuries. Am. J. Occup. Ther. 21(1967)385-395.
37. Weinstein, S. Intensive and extensive aspects of tactile sensitivity as a function of body part, sex, and laterality. In: The Skin Senses. Kenshalo, D. R., ed. Springfield, Illinois: Charles C. Thomas, 1968, pp. 195-212.
1. Ph.D., P.T., Director, Rehabilitation Services, Louisiana State University Health Sciences Center Diabetes Foot Program, 3849 North Boulevard, Baton Rouge, LA70806, U.S.A.
2. Ph.D.,R.P.T., Consultant, The Leprosy Mission International, Green Pastures Hospital, Nepal and Schieffelin Leprosy Research & Training Center, Karigiri, India.
3. R.P.T., Physiotherapist, Department of Hand Rehabilitation, Academisch Ziekenhuis Rotterdam-Dijkzigt, Rotterdam, The Netherlands.
4. O.T., Occupational Therapist Consultant, McKean Rehabilitation Center, Chiang Mai 5000, Thailand.
Reprint requests to Dr. Birke at the above addressor FAX 1-225-342-9777; e-mail: jirke@lsulisc.edu
Received for publication on 2 June 2000.
Accepted for publication in revised form on 1 September 2000.