- Volume 68 , Number 3
- Page: 307–11
Hansen's disease in a patient with a history of sarcoidosis
ABSTRACT
We report a rare case of concomitant Hansen's disease (HD) and sarcoidosis. Reticulin staining may be a helpful diagnostic tool in establishing the diagnosis of sarcoidosis in skin lesions. The diagnosis of HD can be established despite negative polymerase chain reaction results for the detection of Mycobacterium leprae DNA. Finally, a well-established diagnosis of sarcoidosis does not preclude the development of another granulomatous disorder. Hence, when new lesions developed in a patient with sarcoidosis despite appropriate therapy, other concurrent diagnoses should be pursued.RÉSUMÉ
Nous rapportons ici un cas inhabituel concomitant de maladie de Hansen (MH) et de sarcoïdose. La coloration des fibres de réticuline paraitêtre un outils diagnostic utile pour établir le diagnostic de sarcoidose à partir de lésions cutanées. Le diagnostic de MH peut être posé en dépit de résultats négatifs utilisant la technique de polymérase en chaîne pour détecter l'ADN de Mycobacterium leprae. Finalement, un diagnostic de sarcoidose n'écarte pas à priori le développement d'un autre désordre granulomateux. Ainsi, lorsque de nouvelles lésions se développent chez un patient atteint de sarcoïdose en dépit d'une thérapeutique adéquate, une recherche en vue d'autres diagnostics devrait être poursuivie.RESUMEN
Reportamos un caso raro de enfermedad de Hansen (HD) concomitante con sarcoidosis. La tincion de reticulina puede ser util para establecer el diagnostico de sarcoidosis en las lesiones de la piel. El diagnóstico de HD puede ser establecido a pesar de los resultados negativos de la reacción en cadena de la polimerasa para la detección de DNA de Mycobacterium leprae. Finalmente, un diagnóstico bien establecido de sarcoidosis no excluye el desarrollo de otro desorden granulomatoso. Por esto, un paciente con sarcoidosis apropiadamente tratado que desarrolle nuevas lesiones debe estudiarse a fondo para buscar enfermedades concurrentes.Hansen's Disease (HD) and sarcoidosis are both granulomatous disorders which may have similar cutaneous presentations. There have been several reported cases in the literature of sarcoidosis masquerading as HD and vice-versa (2, 4, 9, 11, 15). To our knowledge, there have been no cases of concomitant HD and sarcoidosis. We report here a case of HD developing in a patient with a history of sarcoidosis for many years.
CASE REPORT
A 50-year-old, white Latin woman presented to the University of Miami/Jackson Memorial Hospital Hansen's Disease Clinic (HDC), Miami, Florida, U.S.A., with a 13year history of sarcoidosis and a recent skin biopsy which showed paucibacillary HD. She was born in New York City, lived in Puerto Rico from age 17 to 22, and then lived in the Virgin Islands for 10 years. At age 27, the patient noted a hypopigmented eruption on her ears and left lower eyelid. No diagnosis or treatment was established at that time. At age 31, she received silicone-gel breast implants.
At age 37, the patient was diagnosed with sarcoidosis based on biopsies of the cervical, submental and mediastinal lymph nodes, which revealed noncaseating granulomatous lymphadenitis with numerous lipidized cells, and no organisms were identified with special stains. There were no foreign body giant cells noted. Her angiotensin converting enzyme (ACE) level was normal and her serum calcium level was low. The sedimentation rate was mildly elevated at 20 mm/hr (normal: <20 mm/hr for women). The patient was started on prednisone and hydroxychloroquine sulfate. Her symptoms of fatigue and external dyspnea persisted despite this therapy. At 44 years of age, she had her silicone-gel breast implants removed due to leakage. The same year, a chest X-ray revealed prominent hila bilaterally with possible adenopathy. During the next 4 years, surgical specimens taken from the ethmoids and nasal bone in operations to treat her chronic sinusitis and skin biopsies from both hands revealed noncaseating granulomatous inflammation with no microorganisms detected on special stains. No foreign particles were identified.
At age 48, electromyogram studies revealed a sensory polyneuropathy of the hands and feet for which she was started on gabapentin. One year later, a violaceous, indurated plaque on the right cheek was biopsied by her dermatologist. The specimen, read by the National Ambulatory Hansen's Disease Program (NAHDP), showed replacement of approximately 50% of the dermis by granulomatous inflammatory infiltrates. The granulomas were well formed with a peripheral mantle of lymphocytes. There was necrosis and rare acid-fast bacilli (AFB) were identified. The cutaneous nerves were not well represented in the sample. Polymerase chain reaction (PCR) of this specimen was negative for Mycobacterium leprae. Based on this biopsy, a diagnosis of tuberculoid HD was made and the patient was started on dapsone 100 mg and rifampin 600 mg daily, with slight improvement of her skin lesions. The patient's sarcoidosis medication was discontinued.
In March 1998, after 4 months of dapsone and rifampin therapy, the patient presented to the HDC for a second opinion. She presented with four 3-cm to 5-cm violaceous, indurated hypoesthetic plaques on the cheeks, forehead, and nose; madarosis, bilateral enlarged ulnar nerves, and a mild right ulnar neuritis (Fig. 1). She also had four 2-cm to 3-cm red indurated plaques on the right arm and one on the left hand. All of these lesions had decreased sensation as assessed by the Hand Set Sensory Testing Nylon FilamentCarville®, ranging from residual texture sensation to loss of protective sensation. Biopsy specimens from her right arm and left hand were sent to the NAHDP and revealed replacement of 70%-80% of the dermis by granulomatous inflammatory infiltrates with cutaneous nerve involvement within some granulomas (Fig. 2). Other granulomas had small foci of coagu lative necrosis; however, no AFB were identified in the Fite-stained sections. Due to the large number of skin lesions, the diagnosis was changed to mid-borderline HD, and she was continued on dapsone and rifampin. The patient refused clofazamine due to the pigmentary changes associated with the drug. Five months after beginning multidrug therapy, the patient developed a type 1 reaction with edema and mild paraesthesias of the facial plaques which responded to ibuprofen 400 mg q.i.d. Three months after the initiation of HD medication, the patient agreed to the addition of clofazamine 50 mg q.d. Although her facial eruption became less violaceous on this therapy, she continued to develop new facial lesions.
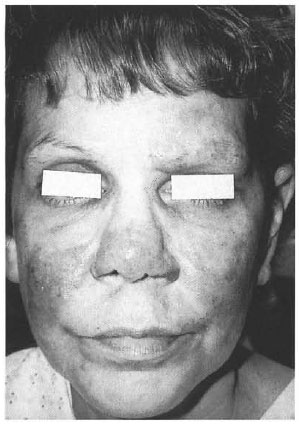
Fig. 1. Violaceous, indurated lesions of thecheeks, nose, and forehead with madarosis.
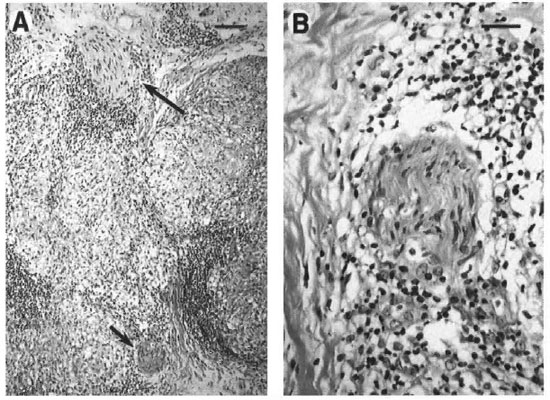
Fig. 2. Granulomatous infiammation of the dermis with involvement of cutaneous nerves. A = Extensive re-placement of the dermis by epithelioid granulomas was observed with involvement of cutaneous nerves in several foci (arrows). B = Closer examination of the nerve indicated by the short arrow in A reveals perineurial andendoneurial infiltration by mononuclear cells, including lymphocytes and vacuolated histiocytes. (A bar = 100µm; B bar = 50 µm.)
A reticulin stain of the left hand skin biopsy in September 1998 showed preservation of the normal reticulin network (Fig. 3). A biopsy of a right nasolabial fold plaque showed noncaseating granulomatous dermatitis consistent with sarcoidosis, with no organisms identified on Fite staining. Slit-skin smears of the earlobes, elbows, and knees were negative for AFB.
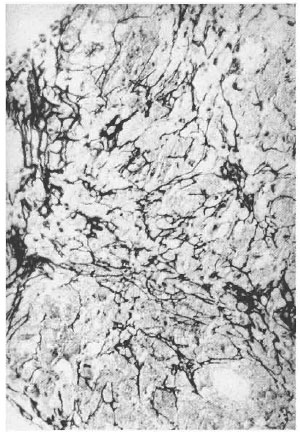
Fig. 3. Reticulin stain showing. intact reticulin network (x10).
A biopsy of a new perioral lesion also revealed noncaseating granulomatous inflammation with no AFB on Fite staining, consistent with sarcoidosis. Slit-lamp examination of the eyes demonstrated clear corneas with normal maculae and no nodules. Periodic ear, nose and throat examinations revealed a chronic sinusitis, clear, open and draining nasal passages with chronic debris, eschar, and friable mucosa. No nasoseptal thinning, ulceration or perforation were evident.
In addition, repeat studies revealed bilateral hilar fullness on chest X-ray and an elevated serum ACE level of 115 U/L (18-52 U/L normal range). It was concluded that the patient had concomitant HD and sarcoidosis. Intralesional triamcinolone acetonide (5 mg/cc) treatment of her facial plaques resulted in decreased induration of these lesions. After 4 months of intralesional steroids, prednisone 10 mg daily was added with further improvement of the induration. The patient has had no progression of her neurosensory loss since multidrug therapy has been instituted.
DISCUSSION
Sarcoidosis is a multisystem disease of unknown etiology. It is characterized by noncaseating granulomas that may involve any organ. The lungs and thoracic lymph nodes are almost always affected, followed by the skin, eyes, and other organs (6). The diagnosis of sarcoidosis is one of exclusion. Other causes of granulomatous inflammation (infectious agents, organic and inorganic agents, neoplasia and autoimmune disorders) need to be ruled out. There are no definitive diagnostic blood, skin, or radiographic tests specific for sarcoidosis. Specific skin lesions in sarcoidosis reveal noncaseating granulomas, and include papules, plaques, nodules, and subcutaneous changes in old scars (16). Laboratory findings may include hypercalciuria, with or without hypercalcemia (6), and elevated serum ACE levels (16). However, serum ACE is elevated in only 60% of patients with sarcoidosis (1,5) and adds little diagnostic value because of its low specificity (1,6).
Hansen's disease is a chronic granulomatous disease with a spectrum of clinical presentations caused by M. leprae. The spectrum of HD ranges from a high cellmediated response to M. leprae in tuberculoid HD to a low cell-mediated response in lepromatous HD. Patients may also present with borderline HD in between the polar forms of the disease (10). The typical clinical features of HD include well-defined erythematous skin lesions and enlarged peripheral nerves with pain and/or sensory loss. Histopathologic evidence of HD includes granulomatous inflammation, AFB, and involvement of cutaneous nerves with foci of necrosis. A surrounding lymphocytic inflammation can often be appreciated. Endemic areas of HD include central Africa, Southeast Asia, and Latin America (16).
Sarcoidosis and HD are both granulomatous disorders that can have similar clinical and histopathological features. Although sarcoidosis is classically described as having noncaseating granulomas, the granulomas of HD are also usually noncaseating. There have been reported cases of difficulty in distinguishing the two disorders (2,4,9,11,15). One case report describes such a diagnostic dilemma in a patient with an erythematous infiltrated facial plaque. Interestingly, the discussion notes that dermatologists in HD-endemic areas would tend to make the diagnosis of HD for a patient with sarcoidosis (4).
Our patient was originally diagnosed with sarcoidosis, and her diagnosis was changed to HD after a facial plaque biopsy revealed granulomatous inflammation and rare AFB. After further work-up, it was concluded that she has both sarcoidosis and HD, based on the clinical and histopathologic findings that were consistent with both disorders. Reticulin staining and PCR were used as diagnostic tools.
Reticulin fibers are composed of Type III collagen, which is a normal component of the dermis. Reticulin fibers are present in granulomas and make up a network that can be seen with special staining (14). In tuberculoid HD there is "patchy destruction and fragmentation of the reticulin" (7). Hence, it is hypothesized that the preservation of the normal reticulin network is consistent with sarcoidosis since the network appears to be disrupted in infectious processes. In our patient there is evidence of sarcoidosis and HD. A biopsy of a plaque on her left hand revealed a preserved reticulin network suggestive of sarcoidosis. The same biopsy also had areas of necrosis within the granulomas which might be consistent with HD.
PCR has been used in the detection of M. leprae in biopsy specimens of patients suspected of having HD lesions. In endemic regions, high sensitivity and specificity have been reported in detecting M. leprae (3). However, in a nonendemic population it is recommended to use PCR in cases with AFB present on microscopy who have atypical histopathologic and clinical features which may obscure the diagnosis (l3). In our patient, PCR of a facial lesion did not detect M. leprae.
It has been suggested that silicone may be responsible for the development of sarcoidosis (17,18). However, the relationship between silicone and sarcoidosis is a controversial issue (8,12). Our patient has a history of silicone-gel breast implants that leaked and were subsequently removed. However, the history of ruptured siliconegel breast implants does not alter the diagnosis of sarcoidosis because the first skin lesions appeared 4 years prior to the placement of the implants. In addition, multiple biopsies from the lymph nodes and skin did not reveal foreign-body-type giant cells.
Acknowledgment. The authors would like to acknowledge Tom Gillis, Ph.D., Chief, Molecular Biology Department, National Hansen's Disease Center at Louisiana State University, Baton Rouge, Louisiana, U.S.A., for performing the polymerase chain reaction on the tissue specimen.
REFERENCES
1. Allen, R, K. A. Angiotensin-converting enzyme. In: Sarcoidosis and Other Granulomatous Disorders. James, D. G., ed. New York: Marcel Dekker Inc., 1994, pp. 529-564.
2. Fields, J. P. and Hellreich, P, D. Sarcoidosis masquerading as Hansen's disease. Arch. Dermatol. 100(1969)649-651.
3. Job, C. K., Jayakumar, J., Williams, D. L. and Gillis, T. P. Role of polymerase chain reaction in the diagnosis of early leprosy. Int. J. Lepr. 65(1997)461-464.
4. Levy, E. and Lantis, S. Sarcoidosis? Leprosy? Arch. Dermatol. 103(1971)349-350.
5. Mana, J., Marcoval, J., Graells, J., Salazar, A., Peyri, J. and Pujol, R. Cutaneous involvement in sarcoidosis; relationship to systemic disease. Arch. Dermatol. 133(1997)882-888.
6. Newman, L. S., Rose, C. S. and Maier, L. A. Sarcoidosis. N. Engl. J. Med. 336(1997)1224-1234.
7. Nirmala, V., Chacko, C. J. and Job, C. K. Tuberculoid leprosy and tuberculosis skin-a comparative histopathological study. Lepr. India 49(1977) 65-69.
8. Pucevich, M. V., Rosenberg, E. W., Bale, G. F. and Terzakis, J. A. Widespread foreign-body granulomas and elevated serum angiotensincoverting enzyme. Arch. Dermatol. 119(1983)229-234.
9. Ramanujam, K. Tuberculoid leprosy or sarcoidosis? A diagnostic dilemma. Lepr. India 54(1982)318-323.
10. Ridley, D. S. and Jopling, W. H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966)255-273.
11. Sawhney, M. P., Srinivasan, S. and Dey, G. N. Leprosy and pulmonary tuberculosis or sarcoidosis? Indian J. Lepr. 67(1995)473-477.
12. Schewach-Millet, M., Ziv, R., Trau, H., Zwas, S. T., Ronnen, M. and Rubenstein, I. Sarcoidosis versus foreign-body granulomas. Int. J. Dermatol. 26(1987)582-585.
13. Scollard, D. M., Gillis, T. P. and Williams, D. L. Polymerase chain reaction assay for the detection and identification of Mycobacterium leprae in the United States. Am. J. Clin. Pathol. 109(1998)642-646.
14. Sheehan, D. C. and Hrapchak, B. B. Theory and practice of histotechnology. In: Connective Tissue and Muscle Fiber Stains. 2nd edn. St. Louis: Mosby, 1980, pp. 181-183.
15. Singh, K., Raina, V., Narulla, A. K. and Singh, R. Sarcoidosis masquerading as leprosy, pulmonary tuberculosis and urolithiasis. J. Assoc. Physicians India 38(1990)657-659.
16. Sugita, Y. Leprosy. Clin. Dermatol. 13(1995)235-243.
17. Val-Bernal, J. F., Sanchez-Quevedo, M. C., Corral, J. and Campos, A. Cutaneous sarcoidosis and foreign bodies; an electron probe roentgenographic microanalytic study. Arch. Pathol. Lab. Med. 119(1995)471-474.
18. Zuk, J. A. and Sims, T. A. Unusual subcutaneous sarcoid-type foreign body granulomatous mass. Histopathology 12(1988)332-334.
1. M.D., M.P.H.; Department of Der-matology and Cutaneous Surgery, University of Miami School of Medicine, Jackson Memorial Hospital, RO.Box 016250 (R-250), Miami, Florida 33101, U.S.A.
2. A. Hendi, M.D.; Department of Der-matology and Cutaneous Surgery, University of Miami School of Medicine, Jackson Memorial Hospital, RO.Box 016250 (R-250), Miami, Florida 33101, U.S.A.
3. M.D.; Department of Der-matology and Cutaneous Surgery, University of Miami School of Medicine, Jackson Memorial Hospital, RO.Box 016250 (R-250), Miami, Florida 33101, U.S.A.
4. R.N., Department of Der-matology and Cutaneous Surgery, University of Miami School of Medicine, Jackson Memorial Hospital, RO.Box 016250 (R-250), Miami, Florida 33101, U.S.A.
5. M.D., Ph.D., GWL Hansen's DiseaseCenter at Louisiana State University, P.O. Box 25072, Baton Rouge, Louisiana, U.S.A.
Reprint requests to Dr. Burdick at the above address or FAX 1-305-243-6468; e-mail: aburdick@med.miami.edu
Received for publication on 25 May 1999.
Accepted for publication in revised form on 15 June 2000.