- Volume 68 , Number 3
- Page: 312–8
Leprosy at a turning point?
Editorial opinions expressed are those of the writers.
We are at a crucial point in the history of leprosy regarding the influence and effect of multiple drug therapy (MDT).
Our attitudes to and handling of leprosy have often been considered referring to three different stages. The clinical picture and the fate of the patients have changed profoundly during the 20th century, from the pre-sulfone era with disfiguring disease to the sulfone era when treatment with dapsone (DDS) induced marked clinical improvement and hope for the future. The situation again became more difficult with the emergence of DDS resistance due to extensive use of monotherapy.
Now, in the era of multiple drug therapy (MDT) the situation is yet another with the elimination of leprosy as a significant public health problem as a declared goal. However, at the beginning of the new millennium we need careful consideration to avoid serious set-backs in a situation in which the dramatic reduction in prevalence may lead us to think that "the leprosy problem" is close to being solved. This is hardly so. The history of tuberculosis control illustrates the dangers of complacency associated with initial success: premature withdrawal of resources from successful control programs in the U.S.A., leading to a resurgence of the disease in the late 1980s.1
Originally, MDT was introduced and advocated by the World Health Organization (WHO) to cope with and prevent further development of drug resistance. Monotherapy with DDS led, as would be expected, to an increasing frequency of DDS resistance.2-4 By the combined use of several drugs with different reaction mechanisms, the risk of drug resistance would be minimized,5-6 leading WHO to recommend standard multiple-drug regimens for treatment of paucibacillary (PB) and muitt.bacillary (MB) leprosy, respectively. With the recommended MDT regimens, patients were shown to soon become noninfectious,6 and the aim of MDT then became to eliminate leprosy as a significant public health problem.
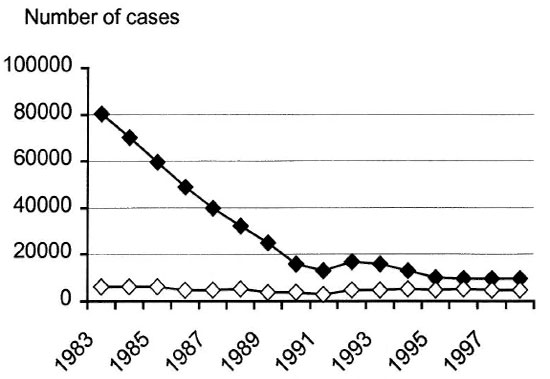
The Figure. Registered leprosy patients in Ethiopia.  = Prevalence;
= Prevalence;  = new cases.
= new cases.
In May 1991, the 44th World Health Assembly adopted Resolution WHA44.9, committing Member States to promote the use of all control measures, including MDT with case finding, in order to attain the global elimination of leprosy as a public health problem by the year 2000. The resolution also urged Member States to maintain political commitment, to strengthen managerial capabilities, to ensure the highest possible level of MDT coverage, to strengthen case finding, to integrate leprosy control within the general health services, to improve information systems, and to coordinate technical and financial resources between international and nongovernmental organizations (NGOs).8
Later it was stated that "This means reducing the proportion of leprosy patients in the community to very low levels, specifically to below one case per 10,000 population."8
After extensive application of MDT for close to 20 years, the pr evalence of leprosy has decreased dramatically, and the number of countries showing prevalence rates above 1 per 10,000 population had been reduced from 122 in 1985 to 32 at the beginning of 1998.8
The Figure shows the number of registered leprosy patients in Ethiopia. A dramatic decrease in prevalence is seen from 1983 to 1996, with the curve then leveling off. Similar curves have been documented in many countries, e.g., from India9 and in the Western Pacific Region.10
Due to changes in definition of the term "leprosy patient," a part of this decline is clearly a statistical feature.
In the days of DDS monotherapy, leprosy patients were kept on the registry for a long time, often beyond cure in PB tuberculoid leprosy.
WHO's "Guide to Eliminating Leprosy as a Public Health Problem"11 states that a case of leprosy is a person with defined clinical signs of leprosy "who has yet to complete a full course of treatment." After completion of MDT the individual is no longer registered as a leprosy patient, even when there are residual disabilities. When only a shorter time period qualifies for the term "leprosy patient," it is evident that the number of registered patients will decrease. How much of the decrease being caused by this statistical feature is probably debatable.
The incidence of infection with Mycobacterium leprae is difficult to assess since M. leprae has a long generation time and the infection develops slowly, often with an insidious onset of clinical symptoms. Indirect methods have to be used, and the number of new cases is often used as a main indicator.
The influence of diagnosis of "hidden cases" needs careful consideration. "Leprosy Elimination Campaigns" (LEC), quite extensively used to promote MDT coverage,13,14 may reveal previously unknown cases, and the registered number of new cases may then be "artificially" higher than the true incidence of disease. In a Leprosy Eradication Drive study in Bombay it is indicated that a decreasing number of new cases may be detected following repeated drives,15 but the accumulated data indicate strongly that the incidence curves have remained flat in several populations extensively covered by MDT for more than a decade. The corresponding statement concerning Table 7 on leprosy trends, 19851997 in WHO's Status Report 1998 on the Action Programme for the Elimination of Leprosy8 is: "At the global level, the leprosy prevalence rate was reduced by 85% and the new case detection rate by only 4%."
For a communicable disease like leprosy, a decline in prevalence while the incidence curves are flat indicates that the infection still goes on at community level, which is a dangerous situation.
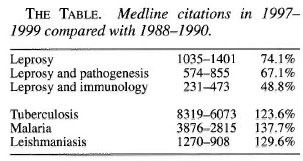
A continuing emphasis on the declining prevalence not taking the flat incidence curves adequately into account may have serious effects: Among them is decreasing funding of leprosy work and a decreasing interest in the disease supported by a belief that the problem is soon to be solved. There is also a definite risk that this will be followed by decreasing knowledge of the disease, resulting in insufficient clinical care, decreasing surveillance in leprosy control programs, and a decreasing research effort. The latter effect is already evident as illustrated in The Table. The number of Medline citations concerning three entries on leprosy were distinctly lower in the 3-year period 1997-1999 than in 1988-1990. By contrast, the Medline search indicates increased research activity regarding other infectious diseases mainly affecting developing countries, tuberculosis, malaria and leishmaniasis.
The current challenge should, therefore, be not only to further promote MDT, but to intensify research and emphasize the importance of careful clinical work. To uphold the ability to recognize early signs of nerve damage in the general health services followed by the institution of adequate treatment and follow up is truly essential: The risk of development of nerve damage within 2 years of initial registration of PB and MB patients can be predicted on the basis of the initial nerve-function loss and their clinical classification.16 Delay in diagnosis with an increased disability score at the start of treatment is an important risk factor for development of serious nerve damage and chronic deformity.
NEED FOR RESEARCH
Transmission of infection. Fundamental aspects concerning mechanisms of transmission of infection with M. leprae are, as far as I understand, unknown!
The current paradigm is that transmission of infection with M. leprae mainly occurs by droplet infection, as in tuberculosis. The significance of MB cases for transmission of infection is evident, as well as the influence of close contacts.17 The relative importance of MB cases for transmission may vary in different populations. PB cases probably account for a significant part of the transmission in societies with a high frequency of this type of infection, as in West Africa. Transmission of subclinical infection certainly needs additional consideration;18 the essential question is rather how important is it than whether it occurs. Again, this may be different under different epidemiological conditions.
Studies of mucosal immunity in leprosy are of great interest in this regard.
Local communities in Ethiopia and India have been selected for study because they are highly endemic for leprosy and have all had extensive application of MDT for more than 10 years. Polymerase chain reaction (PCR) for M. leprae DNA has been performed in nasal swabs at the community level using a primer set specific for this species of mycobacteria. Preliminary findings indicate that M. leprae may be present in these populations without clinical evidence of disease (Smith, W.C.S., personal communication). Ongoing studies seek to clarify the significance of these findings.
Additional information on mechanisms of transmission of the infection has to be obtained to break the transmission and to efficiently control the disease!
Leprosy from an immunological point of view. Since M. leprae is an obligate intracellular parasite, protective immunity depends upon cellular immune reactions mediated by T lymphocytes. T-cell-mediated immune reactions are, however, also responsible for inducting tissue damage.
Regarding tuberculosis, the Koch phenomenon is the classic example of delayedtype hypersensitivity (DTH) inducing tissue damage following infection, illustrating this fundamental dichotomy.19 This dichotomy is typical of leprosy as well, pointing to essential similarities between these two diseases.
After infection with M. leprae only a minority develop clinical symptoms, and the subsequent course depends to a great extent on the immune response of the host.20
The position of the patient in the leprosy spectrum is also mainly determined by the immune response of the host, directly illustrated by the title of Ridley and Jopling's classical paper describing the clinical spectrum of leprosy, "Classification of Leprosy According to Immunity; a Five-Group System."
Immune responses following infection and in different groups within the clinical spectrum need further study. Antibody assays for early detection are particularly effective in the MB forms of the disease, and development of dipstick assays for rapid and simple detection of antibodies under field conditions has been extensively explored. Antibodies to phenolic glycolipid-I (PGL-I) of M. leprae can be demonstrated in whole, finger-prick blood by a simple dipstick assay showing high specificity for infection with M. leprae.22 Further application of these tests in field studies is eagerly awaited. This work needs to be complemented by further development of tests for cell-mediated immunity, now being facilitated by data from the M. leprae genome project.
Vaccine development in tuberculosis is now intensely explored. The interaction with similar work regarding leprosy may be highly rewarding concerning both diseases. Observations made during the recent leprosy vaccine trial in South India represent a challenge to central dogma regarding the effect of vaccination against mycobacterial infections,23 certainly needing further consideration and follow up even in these MDT days.
Affinity of M. leprae for Schwann cells. The particular affinity of M. leprae for Schwann cells is a key feature of this infection. Entirely new information has recently been obtained on this interaction: Firstly, Rambukkana, et al. demonstrated that M. leprae binds to a laminin-α2 dystroglycan bridge present in the basal lamina of Schwann cells in peripheral nerves.24-26
Subsequently, a 21 -kDa histone-like protein (HLP-21) on the M. leprae surface was shown to be its laminin binding receptor,27,28 explaining central features of the homing of M. leprae to peripheral nerves.
However, different mechanisms may be involved in the induction of nerve damage. Induction of nerve damage in the acute stage of reversal reactions is associated with increased cell-mediated immune reactions,29 initiating the view that DTH reactions against antigens released from M. leprae within Schwann cells may lead to damage of the nerve as an innocent bystander.
After the entry of M. leprae into Schwann cells, expression of MHC class II antigens and co-stimulatory molecules is upregulated, making them similar to professional antigen-presenting cells (APC). The Schwann cells may then present M. leprae antigens to nerve-infiltrating, cytotoxic, CD4-positive T cells present in the local environment. This results in the killing of the infected Schwann cells, thus probably being responsible for a major part of the nerve damage leading to the chronic deformities often associated with the disease.30
Other receptor-mediated mechanisms may also be involved in Schwann cells, such as those for the attachment and subsequent entry of M. tuberculosis into macrophages. This area clearly needs further work, in particular to compare the intracellular processing and trafficking of M. tuberculosis and M. leprae after phagocytosis, which is expected, in turn, to affect the type and intensity of immune responses after infection and the eventual tissue damage.
Reactions. Reactions in leprosy fall into two major groups-reversal reactions (RR, type 1) and erythema nodosum leprosum (ENL, type 2)-mediated by different immunological mechanisms. The influence of cytokines on the clinical picture of reactions is striking, with different cytokine patterns being found during stable disease compared with reactional states,31 observations again pointing to leprosy as a model disease to study the influence of immune regulation after infection on the clinical pattern of disease. Tumor necrosis factor-alpha (TNF-α) is of particular interest, clearly being required for protection in mycobacterial infections, too much of it being associated with tissue damage.32-35
Another feature of leprosy reactions appears to be even more significant: the frequent occurrence in connection with MDT; particularly during the first 6 months of treatment, reversal reactions may occur in as much as 80% of patients with borderline leprosy.36 In lepromatous leprosy, ENL-type reactions with progressive nerve damage may occur after completion of the full course of MDT. Emphasizing that they are noninfectious, they are no longer registered as leprosy patients according to the WHO recommendations, but the disease is active. There must be antigen around, stimulating the immune system in bouts. These late reactions should be intensely investigated through combined clinical and immunological studies to delineate pathogenetic mechanisms! Development of tests for early detection and/or prediction of reactions would also be essential for control programs.
The M. leprae genome. The genome of M. tuberculosis has been fully sequenced,37 and a wealth of information has already been obtained from it.38,39 The M. leprae genome has also been completed, but the basic analysis of it has not yet been published. Compared with M. tuberculosis, the M. leprae genome is smaller, with 4,411,529 and 3,268,182 base pairs, respectively, and there is evidence of extensive gene inactivation in M. leprae.40
In earlier studies we found extensive similarities between secreted antigens in M. tuberculosis and M. leprae.41,42 Detailed comparison between the two genomes will be essential for further study of immune responses in these infections, to develop new diagnostic reagents, and to understand the physiology of the two microorganisms. This information on genome structure will be fundamental to explain why M. tuberculosis is one of the most successful human pathogens while M. leprae grows more slowly, only being able to establish and to continue transmission of infection at the community level under particular socioeconomic conditions with extensive poverty.
CONCLUSION
The introduction and extensive application of MDT have had a profound influence on leprosy, on clinical care, and on control work. However, several problems remain. Rather than tending to be swept under the carpet,43 they should be actively identified and openly discussed to be managed.
- Morten Harboe, M.D., Ph.D.
Institute of Immunology
The National Hospital
N-0023 Oslo, Norway and
Armauer Hansen Research Institute (AHRI)
Addis Ababa, Ethiopia
REFERENCES
1. Young, D. Leprosy: a post-elimination research agenda. Trends Microbiol. 6(1998)217-218.
2. Pettit, J. and Rees, R. Sulphone resistance in leprosy; an experimental and clinical study. Lancet 2(1964)673-674.
3. Pearson, J. M., Haile, G. S. and Rees, R. J. Primary dapsone-resistant leprosy. Lepr. Rev. 48(1977)129-132.
4. Pearson, J. M., Haile, G. S., Bametson, R. St.C.and Rees, R. J. Dapsone-resistant leprosy in Ethiopia. Lepr. Rev. 50(1979)183-199.
5. Ji, B. and Grosset, J. H. Recent advances in the chemotherapy of leprosy. Lepr. Rev. 61(1990)313-329.
6. Jacobson, R. Treatment of leprosy. In: Leprosy. 2nd edn. Hastings, R. C., ed. Edinburgh: Churchill Livingstone, 1994,317-349.
7. WHO Study Group. Chemotherapy of Leprosy for Control Programmes. Geneva: World Health Organization, 1982. Tech. Rep. Ser. 675.
8. WHO Action Programme for the Elimination of Leprosy. Status report 1998. Geneva: World Health Organization, 1998.
9. Dharmshaktu, N. S., Barkakaty, B. N., Patnaik, P.K. and Arif, M. A. Progress towards elimination of leprosy as a public health problem in India and role of modified leprosy elimination campaign. Lepr. Rev. 70(1999)430-439.
10. Smith, W. C. Future scope and expectations: why, when, and how LECs should continue. Lepr. Rev. 70(1999)498-505.
11. World Health Organization. Guide to eliminating leprosy as a public health problem. 2nd edn. pocket version. Geneva: World Health Organization, 1997. WHO/LEP/97.7
12. Smith, W. C. We need to know what is happening to the incidence of leprosy. Lepr. Rev. 68(1997)195-200.
13. WHO Action Programme for the Elimination of Leprosy. Guidelines for carrying out leprosy elimination campaigns 1996. Lepr. Rev. 70(1999)408-427.
14. Leprosy elimination campaigns(LEC). Lepr.Rev. 70(1999)404-407.
15. ls Ganapati, R., Revankar, C. R., Bulchand, H. O. and Kingsley, S. The Dharavi storysaga of LECs over 2 decades. Lepr. Rev. 70(1999)495-497.
16. Croft, R. P., Nicholls, P. G., Steyerberg, E. W., Richardus, J. H. and Smith, W. C. S. A clinicai prediction rule for nerve-function impairment in leprosy patients. Lancet 355(2000)1603-1606.
17. van Beers, S. M., Hatta, M. and Klatser, P. R. Patient contact is the major determinant in incident leprosy: implications for future control. Int. J. Lepr. 67(1999)119-128.
18. Cree, I. A. and Smith, W. C. Leprosy transmission and mucosal immunity: towards eradication? Lepr. Rev. 69(1998)112-121.
19. Rook, G. A. W. and Bloom, B. R. Mechanisms of pathogenesis in tuberculosis. In: Tuberculosis; Pathogenesis, Protection and Control. Bloom, B. R., ed. Washington, D.C.: ASM Press, 1994, pp. 485-501.
20. Harboe, M. Overview of host-parasite relations. In: Leprosy. 2nd edn. Hastings, R. C., ed. Edinburgh: Churchill Livingstone, 1994, pp. 87-112.
21. Ridley, D. S. and Jopling, W. H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966)255-273.
22. Buhrer-Sekula, S., Cunha, M. G., Ferreira, W. A. and Klatser, P. R. The use of whole blood in a dipstick assay for detection of antibodies to Mycobacterium leprae: a field evaluation. FEMS Immunol. Med. Microbiol. 21(1998)197-201.
23. Fine, P. E. South Indian leprosy vaccine trial: important lessons for mycobacterial immunology. Lepr.Rev. 70(1999)247-249.
24. Rambukkana, A., Salzer, J. L., Yurchenco P Dand Tuomanen, E. I. Neural targeting of Mycobacterium leprae mediated by the G domain of the laminin-alpha2 chain. Cell 88(1997)811-821.
25. Rambukkana, A., Yamada, H., Zanazzi, G., Mathus, T., Salzer, J. L., Yurchenco, P. D., Campbell, K. P. and Fischetti, V. A. Role of alpha-dystroglycan asa Schwann cell receptor for Mycobacterium leprae. Science 282(1998)2076-2079.
26. Rambukkana, A. How does Mycobacterium leprae target the peripheral nervous system? Trends Microbiol. 8(2000)23-28.
27. Shimoji, Y., Ng, V., Matsumura, K., Fischetti, V. A. and Rambukkana, A. A 21-kDa surface protein of Mycobacterium leprae binds peripheral nerve laminin-2 and mediates Schwann cell invasion. Proc. Natl. Acad. Sc. U.S.A. 96(1999)9857-9862.
28. Pessolani, M., Marques, M., Antonio, V., Sarno, E. and Brennan, P. Characterization of Mycobacterium leprae laminin binding proteins: a candidate adhesin involved in bacterial attachment to Schwann cells. Fourth International Conference on the Pathogenesis of Mycobacterial Infections. Stockholm, 1999, p. 130.
29. Bjune, G., Barnetson, R. St.C., Ridley, D.S. and Kronvall, G. Lymphocyte transformation test in leprosy; correlation of the response with inflammation of lesions. Clin. Exp. Immunol. 25(1976)85-94.
30. Spierings, E., de Boer, T., Zulianello, L., Adams, L., Mommaas, M., Marani, E. and Ottenhoff, T. H. M. Mycobacterium leprae specific, HLA class II restricted killing of human Schwann cells by CD4+ T helper-1 cells: a novel immunopathogenic mechanism of nerve damage in leprosy.(Submitted for publication).
31. Sreenivasan, P., Misra, R. S., Wilfred, D. and Nath, I. Lepromatous leprosy patients show T helper 1-like cytokine profile with differential expression of interleukin-10 during type 1 and 2 reactions. Immunology 95(1998)529-536.
32. Rook, G. A., Attiyah, R. A. and Foley, N. The role of cytokines in the immunopathology of tuberculosis, and the regulation of agalactosyl IgG. Lymphokine Res. 8(1989)323-328.
33. Khanolkar-Young, S., Rayment, N., Brickell, P.M., Katz, D. R., Vinayakumar, S., Colston, M. J. and Lockwood, D. N. Tumour necrosis factor-alpha(TNF-alpha)synthesis is associated with the skin and peripheral nerve pathology of leprosy reversal reactions.Clin. Exp. Immunol. 99(1995)196-202.
34. Barnes, P. F., Chatterjee, D., Brennan, P. J., Rea,T. H. and Modlin, R. L. Tumor necrosis factor production in patients with leprosy. Infect. Immun. 60(1992)1441-1446.
35. Sampaio, E. P., Kaplan, G., Miranda, A., Nery, J.A., Miguel, C. P., Viana, S. M. and Sarno, E. N. The influence of thalidomide on the clinicai and immunologic manifestation of erythema nodosum leprosum. J.Infect. Dis. 168(1993)408-414.
36. Roche, P. W., Theuvenet, W. J. and Britton, W. J.Risk factors for type-1 reactions in borderline leprosy patients. Lancet 338(1991)654-657.
37. Cole, S. T., Brosch, R., Parkhill, J., Garnier, T., Churcher, C., Harris, D., Gordon, S. V., Eiglmeier, K., Gas, S., Barry, C. E., III, Tekaia, F., Badcock, K., Basham, D., Brown, D., Chillingworth, T., Connor, R., Davies, K., Devlin, T., Feltwell, T., Gentles, S., Hamlin, N., Holroyd, S., Hornsby, T., Jagels, K. and Bar-rell, B. G. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393(1998)537-544.
38. Young, D. B. Blueprint for the white plague. Nature 393(1998)515-516.
39. Tekaia, E, Gordon, S. V., Gamier, T., Brosch, R., Barrell, B. G. and Cole, S. T. Analysis of the proteome of Mycobacterium tuberculosis in silico. Tuberc. Lung Dis. 79(1999)329-342.
40. Cole, S. T. Personal communication.
41. Harboe, M. and Wiker, H. G. Secreted proteins of Mycobacterium leprae. Scand. J. Immunol. 48(1998)577-584.
42. Harboe, M. and Wiker, H. G. Searching for secreted proteins of Mycobacterium leprae. Indian J. Lepr. 71(1999)19-35.
43. Harboe, M. Structure of the 15th International Leprosy Congress. Int. J. Lepr. 67(1999)64-65.
* Based on Keynote Address "Leprosy at a Turning Point?" given at the XV International Congress for Tropical Medicine and Malaria, Cartagena, Colombia, 21 August 2000.