- Volume 68 , Number 3
- Page: 319–23
Development of TI leprosy in a BCG-vaccinated individual: immunological response during disease and after spontaneous healing
This department is for the publication of informal communications that are of interest because they are informative and stimulating, and for the discussion of controversial matters. The mandate of this Journal is to disseminate information relating to leprosy in particular and also other mycobacterial diseases. Dissident comment or interpretation on published research is of course valid, but personality attacks on individuals would seem unnecessary. Political comments, valid or not, also are unwelcome. They might result in interference with the distribution of the Journal and thus interfere with its prime purpose.
To the Editor:
In the last decade, several studies have clearly demonstrated a protective effect of BCG (Bacille Calmette-Guérin) vaccination against the development of leprosy (4). Results of a large vaccine trial indicated that several doses of BCG offer additional protection, and that the use of killed Mycobacterium leprae did not improve the protective effect of BCG alone (3). In leprosy, 1 Thl-type response observed in the tuberculoid forms (TT/BT) has been associated with a high gamma interferon (IFNγ) production and development of effective cell-mediated immunity resulting in elimination of the bacilli. As described recently, BCG vaccination in humans was able to induce a Thl response and enhanced IFNγ production to specific antigens (12) and to M. leprae antigens as well (10). Thus, the protection afforded by BCG seems to be related, at least in part, to the induction of IFNγ secretion following vaccination. In the present study, clinical and immunological follow-up evaluation of a household contact who developed TT leprosy after BCG vaccination was assessed during active disease and after self-healing. A major participation of CD4+ T cells in the in vitro response is further demonstrated.
Clinical and immunological response. In her first clinical examination at the Leprosy Outpatient Unit (Oswaldo Cruz Foundation, Rio de Janeiro, Brazil), a healthy household contact, aged 55, was vaccinated with 0.1 ml BCG (Moreau strain, Ataulpho de Paiva Foundation, Rio de Janeiro, Brazil) administered intradermally in accordance with the National Leprosy Program recommendation. Approximately 4 months after BCG vaccination, the contact returned to the clinic due to the appearance of skin lesions on her face and right upper limb that had become visible 2 months after vaccination. The lesions resembled the polar tuberculoid form similar to those seen in children and commonly referred to as infantum nodular leprosy (INL). This individual presented with a positive lepromin skin test (22 mm with central necrosis) and a negative bacterial index. A clinical and histological diagnosis of TT leprosy was established (13) and immunostaining of tissue biopsy using antiCD3 antibody showed a large lymphocytic infiltrate in which more than 50% of the CD3+ T cells were also CD4+ cells, and less than 10% were CD8+. Although the patient had regular clinical and immunological follow-up examinations every 3 months no treatment was given (as recommended for INL) and progressive involution of the lesions was noted. Sixteen months later, all lesions had disappeared, showing atrophic scars similar to those that occur in INL. After 1 year, even these scars were not detected.
The immunological response in vitro to whole M. leprae (20 µg/ml) at the time of leprosy diagnosis showed a strong lymphoproliferative response (LTT, Δcpm ± S.D. = 10,414 ± 1202) and detectable IFNγ levels (860 pg/ml) as measured by ELISA. Once the patient developed an INL-like form following BCG vaccine, the cytokine profile secreted by peripheral blood mononuclear cells (PBMC) obtained at different periods during the follow up was investigated. Upon stimulation with M. leprae or BCG (20 p,g/ml), very high levels of IFNγ (10,090 and 801 pg/ml, respectively) and tumor necrosis factor-alpha (TNFα) (157 and 6.2 ng/ml) were detected during active disease (a period corresponding to 7 months after BCG vaccination and 3 months after leprosy diagnosis). In contrast, IL-5 (M. leprae = 317 pg/ml; BCG = 125 pg/ml) and IL-10 levels (M. leprae = 478 pg/ml; BCG = 2016 pg/ml) were in the range observed for healthy leprosy household contacts (10). Moreover, IL-2 production following M. leprae stimulation was 3950 cpm as measured in the CTLL bioassay (6). Additional experiments were then carried out to determine whether mycobacteria-reactive Thl-like T cells were able to recognize a panel of proteins purified from the mycobacteria. The purification of mycobacterial antigens was as described by Hunter, et al. (8) and Pessolani, et al (11). A prominent response (IFNγ production >100 pg/ml) was detected to antigens shared by BCG and M. leprae (85 complex, GroEL/10 kDa and BFR/22 kDa) as well as to the M. leprae specific antigen, the MMP-I/35 kDa protein (not shown).
Assuming that the intense Thl-like response evoked by BCG vaccination could at least be partially responsible for the appearance of leprosy lesions, we monitored cytokine levels after complete regression of the patient's lesions (month 16 of follow up) as compared to the levels detected during active disease (month 7 of follow up). As expected (Fig. 1), a 53% and 84% decrease in the capacity of PBMC to secret TNF-α and IFNγ, respectively, in response to M. leprae was observed after healing.
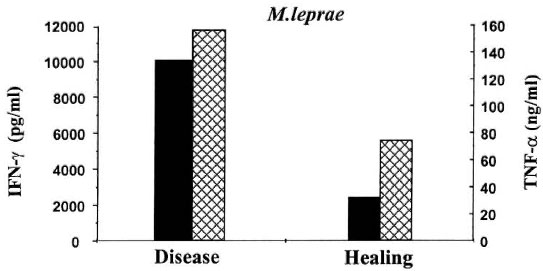
Fig. 1. In vitro immunological response of one TT leprosy patient during active disease (7 months of follow up) and after healing of leprosy (16 months of follow up). PBMC (1 x 10 cells) were stimulated with M. leprae for 5 days in 24-well culture plates. Cytokine leveis in cell-free culture supernatants (24 hr culture for TNF-α; 5day culture for IFN-γ) were determined using specific ELISA (Pharmingen).  = IFN-γ;
= IFN-γ;  = TNF-α.
= TNF-α.
To identify the subpopulation of T cells recognizing M. leprae, PBMC were depleted of CD4+ (PBMC-CD4) or CD8+ (PBMC-CD8) T cells by negative selection using magnetic beads (Bio Mag, Cambridge, Massachusetts, U.S.A.), according to the manufacturer's recommendation. Flow cytometry analysis and functional assays (LTT and cytokine measurement) were performed before and after T-cell depletion. As expected, the positive LTT seen in response to M. leprae (Δcpm = 7727 ± 1777) in the PBMC population was reduced when CD4+ T cells (3385 ± 300), but not CD8 (12,053 ± 3223), were depleted. In addition, reductions in IFNγ production of 66% and 86% was reached in PBMC-CD4 cultures stimulated with M. leprae and BCG, respectively. Accordingly, no significant effect on IFN-γ was noted in response to M. leprae when CD8+ cells were depleted from the cultures (880 pg/ml in the PBMC versus 681 pg/ml in the PBMC-CD8 population). Analysis of IL-10 secretion in these cultures was also assessed. Interestingly, slight increases in IL-10 production were observed in response to M. leprae (Fig. 2) when both CD4 and CD8 T-cell populations were depleted.
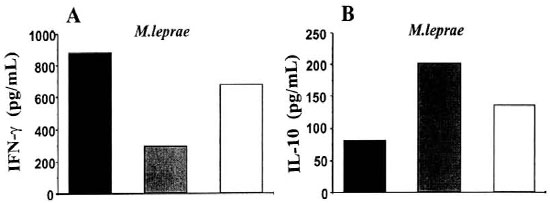
Fig. 2. PBMC depleted of the CD4+ T lymphocytes showed decreased IFN-γ secretion in response to M.leprae when compared with whole PBMC (19 months of the follow-up period). PBMC were depleted of theCD4+ or CD8+ T lymphocytes and stimulated with M. leprae (20 µg/mi). Supematants were harvested and leveisof IFN-γ (A) and IL-10 (B) were determined by ELISA.  = PBMC;
= PBMC;  = -CD4
= -CD4  ; = -CD8.
; = -CD8.
It was interesting that, in our clinic, another three household contacts (one of them was the grandchild of the TT patient) have also developed tuberculoid leprosy after BCG vaccination (they also presented positive LTT and IFNγ release in response to M. leprae in vitro), supporting the hypothesis, in accordance with others (1), that BCG vaccine could shift the immune response to the tuberculoid side of the leprosy spectrum. In order to determine the human histocompatibility leukocyte antigen (HLA) haplotype inherited by these two family members who developed leprosy following BCG vaccination, the family segregation of class I and class II molecules was analyzed using serological HLA typing by the microlymphocytoxicity method (14). Our findings defined the TT patient as A2, A31, B35, B51, Cw4, , DR16, DR51, DQ7 (for the grandchild it was A2, A23, B35, B62, Cw2, Cw4, DR7, DR13, DR52, DR53, DQ2, DQ6). Therefore, there can be two different HLA associations contributing to the disease in this family, since they exhibited a split of HLA-DQ1 (DR16 and DQ6) and the HLA-DR13.
The ability of viable (vaccine) but not killed BCG to induce IFNγ early in infection has been reported to play a critical role in the generation of protective antigen-specific T-cell response against BCG (5). Immunological follow up of household contacts who were BCG vaccinated demonstrated enhanced IFNγ production and activation of an anti-inflammatory loop with increased IL-10 release and concomitant decrease in TNF-a production following stimulation of PBMC with whole M. leprae in vitro (10). Possibly, in this patient who presented with INL, the high levels of IFNγ and TNF-a were related to the development of her leprosy lesions. The concomitant increase in IL-10 could have led to further downregulation of cytokine production in vitro which paralleled deactivation of the immune inflammatory response during self-healing of her leprosy.
In conclusion, the immunological follow up of a household contact who developed TT leprosy following BCG vaccination demonstrated a preferential Thl-type response during active disease. The enhanced levels of cytokines detected during the disease were reduced after self-healing, suggesting that at this moment an equilibrium between proand anti-inflammatory cytokines has been established with emergence of a ThO response, as observed previously by Lima, et al. (10). Indeed, the major T-cell subset involved in M. leprae responsiveness was shown to be the CD4+ T cells. It is likely that, in this patient, vaccination with BCG led to amplification of the inflammatory response resulting in an imbalance between proand anti-inflammatory cytokines, culminating with disease manifestation with hyper-responsiveness. Our findings and those of others (2) suggest that early and late responses to mycobacterial infection are influenced by previous exposure to vaccination and/or environmental mycobacteria in addition to genetic factors (7).
- Monica Cristina B. S. Lima, Ph.D.
Leprosy Laboratory
Oswaldo Cruz Foundation
Rio de Janeiro, RJ, Brazil and
Laboratory of Immunopathology
The Roberto Alcantara Gomes Institute of Biology
State University of Rio de Janeiro
Rio de Janeiro, RJ, Brazil
- Jorge L. Salgado, Ms.C.
Maria C. V. Pessolani, Ph.D.
Leprosy Laboratory
Oswaldo Cruz Foundation
Rio de Janeiro, RJ, Brazil
-Geraldo M, B. Pereira, M.D., Ph.D.
Leprosy Laboratory
Oswaldo Cruz Foundation
Rio de Janeiro, RJ, Brazil and
Laboratory of Immunopathology
The Roberto Alcantara Gomes Institute of Biology
State University of Rio de Janeiro
Rio de Janeiro, RJ, Brazil
- Franklin D. Rumjanek, Ph.D.
Biochemistry Department
Federal University of Rio de Janeiro
Rio de Janeiro, RJ, Brazil
- Nadia Duppre, Ms.C.
Jose A. C. Nery, M.D., Ms.C.
Leprosy Laboratory
Oswaldo Cruz Foundation
Rio de Janeiro, RJ, Brazil
- Luis C. M. S. Porto, M.D., Ph.D.
Luciane F. S. Pontes, Ms.C.
School of Medical Sciences and
Laboratory of Histocompatibility
The Roberto Alcantara Gomes Institute of Biology
State University of Rio de Janeiro
Rio de Janeiro, RJ, Brazil
- Euzenir N. Sarno, M.D., Ph.D.
Elizabeth P. Sampaio, M.D., Ph.D.
Leprosy Laboratory
Oswaldo Cruz Foundation
Rio de Janeiro, RJ, Brazil
REFERENCES
1. Chaudhury, S., Hazra, S. K., Saha, B., Mazumder, B., Biswas, P. C., Chattopadhya, D. and Saha, K. An eight-year field trial on antileprosy vaccines among high-risk household contacts in the Calcutta metropolis. Int. J. Lepr. 62(1994)389-394.
2. Cheng, S. h., Walker. K. B., Lowrie, D. B., Mitchison, D. A., Swamy, R., Datta, M. and Prabhakar, R. Monocyte antimycobacterial activity before and after Mycobacterium bovis BCG vaccination in Chingleput, India, and London, United Kingdom. Infect. Immun. 61(1993)4501-4503.
3. Convit, J., Sampson, C., Zuninga, M., Smith, P. G., Plata, J., Silva, J., Molina, J., Pinardi, M. E., Bloom, B. R. and Salgado, A. Immunoproprylactic trial with combined Mycobacterium, leprae/BCG vaccine against leprosy: preliminary results. Lancet 1(1992)446-450.
4. Fine, P. E. M. Implications of genetics for the epidemiology and control of leprosy. Phil. Trans. R. Soc. Lond. B321(1988)365-376.
5. Flesch, I. E., Hess, J. H., Huang, S., Aguet, M., Rothe, J., Bluethmann, H. and Kaufmann, S. H. Early interleukin 12 production by macrophages in response to mycobacterial infection depends on interferon gamma and tumor necrosis factor alpha. J. Exp. Med. 181(1995)1615-1621.
6. Gillis, S., Perm, W., Ou, W. and Smith, K. A. T cell growth factor: parameters for production and a quantitative micro assay for activity. J. Immunol. 120(1978)2027-2032.
7. Hill, A. V. The immunogenetics of human infectious diseases. Ann. Rev. Immunol. 161(1998)593-617.
8. Hunter, S. W., Rivoire, B., Mehra, V., Bloom, B. R. and Brennan, P. J. The major native proteins of the leprosy bacillus. J. Biol. Chem. 265(1990)14065-14068.
9. Launois, P., Niang, M. N., De Bruyn, J., Sarthou, J. L., Rivier, F., Drowart, A., Van Vooren, J. P., Millan, J. and Huygen, K. The major secreted antigen complex(Ag 85)from Mycobacterium bovis bacille Calmette-Guerin is associated with protective T cells in leprosy: a follow-up study of 45 household contacts. J. Infect. Dis. 167(1993)1160-1167.
10. Lima, M, C. B, S., Pereira, G. M. B., Rumjanek, F. D., Gomes, H. M., Duppre, N., Sampaio, E. P., Alvim, I., Nery, J. A. C., Sarno, E. N. and Pessolani, M. C. V, Immunological cytokine correlates of protective immunity and pathogenesis in leprosy. Scand. J. Immunol. 51(2000)419-128.
11. Pessolani, M. C., Peralta, J. M., Rumianek, F. D., Gomes, H. M., Marques, M. A., Almeida, E. C., Saad, M. H. and Sarno, E. N. Serology and leprosy: immunoassays comparing immunoglobulin G antibody responses to 28and 30-kilodalton proteins purified from Mycobacterium bovis BCG. J. Clin. Microbiol. 29(1991)2285-2290.
12. Ravn, P., Boesen, H., Pedersen, B. K. and Andersen, P. Human T cell responses induced by vaccination with Mycobacterium bovis bacillus CalmetteGuerin. J. Immunol. 158(1997)1949-1955.
13. Ridley, D. S. and Jopling, W. H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966) 255-273.
14. Terasaki, P. I. and McClelland, J. D. Microdroplet assay of human serum cytotoxins. Nature 204(1964)998-2000.
Reprint requests to Elizabeth P. Sampaio, M.D., Ph.D., Leprosy Laboratory, Oswaldo Cruz Foundation, Av. Brasil 4365, Manguinhos, 21045-900 Rio de Janeiro, RJ, Brazil or FAX 55-21-270-9997.