- Volume 68 , Number 3
- Page: 272–6
Relapse as histoid leprosy after receiving multidrug therapy (MDT); a report of three cases
ABSTRACT
The histoid type of leprosy has been described as occurring in lepromatous leprosy patients who relapse after many years of apparently successful dapsone monotherapy. Three patients who had received the World Health Organization-recommended regimens of multidrug therapy (WHO/ MDT) relapsed as histoid leprosy 12-15 years after completion of treatment. In one patient, through mouse foot pad studies, the bacilli were found to be sensitive to rifampin and clofazimine and resistant to dapsone. In the other two patients mouse foot pad studies were inconclusive. The patients were re-started on WHO/ MDT. Two patients took regular treatment and improved, both clinically and bacteriologically. One patient was irregular in treatment, and 1 year after re-starting WHO/ MDT nodules were still present although the bacterial index had fallen slightly.RÉSUMÉ
La lèpre de type histoïde a été originellement décrite chez des patients souffrant originellement de lèpre lëpromateuse qui ont rechuté après plusieurs années de rémission suivant une mono thérapie à la dapsone. Trois patients qui avaient reçu un traitement de poly chimiothérapie recommandée par l'organisation mondiale de la santé (OMS/PCT) ont rechuté sous forme d'une lèpre histoïde 12 à 15 années après la fin du traitement. Chez un patient, à l'aide d'études d'inoculation à la plante des pattes de souris, les bacilles furent démontrés être sensibles à la rifampicine, la clofazimine et résistantes à la dapsone. Chez les autres patients, les études d'inoculation aux plantes des pattes de souris se sont révélées non concluantes. Un traitement OMS/ PCT fur ré-institué chez ces patients. Deux patients qui prirent régulièrement leur traitement s'améliorèrent à la fois cliniquement et bactériologiquement. Un patient fit un traitement irrégulier et un an après la reprise du traitement, des nodules étaient encore présents avec un indice bactérioscopique légèrement diminué.RESUMEN
El tipo histoide de la lepra se ha descrito en pacientes lepromatosos que muestran recaída después de muchos anos de tratamiento aparentemente exitoso con dapsona. Très pacientes que habian recibido la poliquimioterapia recomendada por la Organización Mundial de la Salud (PQT/OMS) recayeron con lepra histoide 12 a 15 anos después de completar el tratamiento. En un paciente, los bacilos fueron sensibles a la rifampina y clofazimina, y resistentes a la dapsona en el modelo de la almohadilla plantar del ratón. En los otros dos pacientes los resultados no fueron concluyentes. Los pacientes fueron vueltos a tratar con la PQT/ OMS. Dos pacientes tomaron el tratamiento de manera regular y mejoraron clínica- y bacteriológicamente. Un paciente fue irregular en su tratamiento y un ano después de reiniciar el tratamiento con PQT/OMS todavia presentaba nódulos, aunque el índice bacteriano habia disminuido ligeramente.Histoid leprosy is an unusual variant of lepromatous leprosy. It was first described by Wade in 1960 (13). It is a well-recognized entity, having characteristic skin lesions, histopathology and bacterial morphology. It occurs in patients on long-term dapsone monotherapy, showing initial improvement followed by relapse (15,7). Irregular and inadequate therapy with dapsone (8,9) and the development of dapsone resistance (2,5,9) are thought to be important for the development of histoid leprosy. It has also been reported in untreated leprosy patients (6,9).
Here we report three cases of relapse as histoid leprosy in patients who had received multidrug therapy (MDT) for 2 years. All three patients were part of the THELEP- sponsored field trials of MDT in multibacil- lary (MB) leprosy patients which began at Schieffelin Leprosy Research and Training Centre (SLR&TC) in Karigiri, India, in December of 1981. Two regimens, each consisting of dapsone, rifampin and clofazimine, were used to treat the patients.
CASE REPORTS
Case No. 1. A 10-year-old boy was diagnosed as a case of lepromatous leprosy in April 1968. A skin-smear examination from the routine sites (routine sites for skin smear at SLR&TC are right earlobe, left forehead, right chin and left buttock or left thigh) showed a bacterial index (BI) of 3.5+, and a skin biopsy from a patch on his back was consistent with lepromatous leprosy.
He was treated with dapsone monotherapy for 14 years. His BI decreased gradually, and he became skin-smear negative in 5 years. Treatment with dapsone was continued. In June 1982, during the 14th year of dapsone monotherapy, after remaining skin-smear negative for 9 years, he again became skin-smear positive with a mean BI of 0.75+. However, clinically he was still inactive. He was lepromin negative. He received conventional MDT (WHO/MDT) consisting of monthly supervised pulses of 600 mg rifampin and 300 mg clofazimine along with a daily unsupervised dosage of 50 mg clofazimine and 100 mg dapsone. After 2 years, he was released from treatment (RFT). At the time of RFT he was clinically inactive and his skin smears from the routine sites were negative for acid-fast bacilli (AFB). He was followed up at least once a year after RFT when clinical and bacteriological assessments were done. Eleven years after RFT he was lost for follow up.
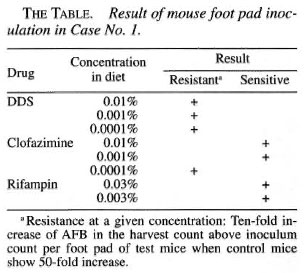
He presented again in August of 1998, 15 years after completion of MDT, with nontender, firm, shiny nodules on the cheeks, each about 1.5 x 1.5 cm. The BI from one of the nodules on the left cheek was 5+; his overall BI was 1.25+. A biopsy of a nodule showed features of nodular lepromatous leprosy with histoid areas (Figs. 1, 2, 3). On mouse foot pad inoculation the bacilli were found to be fully resistant to dapsone and to the lowest concentration of clofazimine (0.0001%) (The Table).
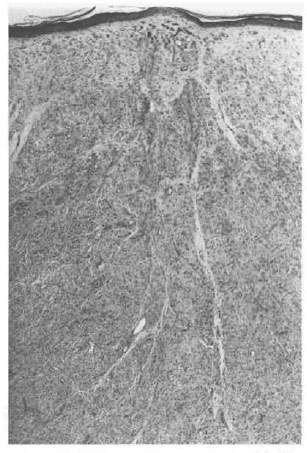
Fig. 1. Photomicrograph showing epidermal atrophy. The dermis is replaced by macrophages arrangedin a nodular pattern (H&E x40).
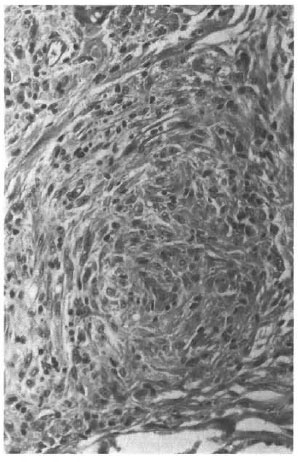
Fig. 2. Photomicrograph showing spindle-shaped macrophages arranged in a whorl (H&Ex200).
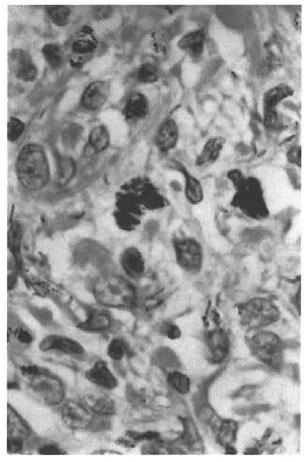
Fig. 3. Photomicrograph showing clumps ofacid-fast bacilli within the macrophages (modifiedFite-Faraco stain x1000).
He was re-started on WHO/MDT, and his nodules regressed and disappeared after 8 months of therapy. After 1 year of MDT he was clinically inactive. His skin smears from the routine sites were negative for AFB, although a selective skin smear from the left cheek was positive with a BI of 2+. His initial BI at this site was 5+.
Case No. 2. A 16-year-old male presented in March 1977 with multiple, ill-defined, asymmetrically distributed anesthetic patches and nerve thickening. A skin-smear examination showed a BI of 2+. He was clinically diagnosed as a case of borderline lepromatous (BL) leprosy and was started on dapsone monotherapy which he received irregularly for a period of 1 year, after which he discontinued treatment.
Six years later, in February 1984, he presented with thickening of the earlobes, supraciliary madarosis, diffuse infiltration of the skin all over the body, glove-and- stocking anesthesia, a few nodules on the buttocks, and an area with patchy loss of sensation on the right thigh. Both ulnar and both radial cutaneous nerves were thickened. Skin smears from routine sites showed a mean BI of 3.75+. He was lepromin negative and the skin biopsy done from a nodule on his right buttock was consistent with borderline lepromatous leprosy.
He received MDT consisting of monthly supervised doses of 600 mg rifampin, 300 mg clofazimine on two consecutive days, bimonthly injections of 225 mg of acedap- sone, and a daily unsupervised dose of 100 mg dapsone. After 2 years he was released from treatment (RFT), at which time his BI was 3.25+. He was followed up every year at which time a clinical examination was done and skin smears from routine sites were taken. His skin smears gradually became negative 5 years after RFT.
Twelve years after RFT, in October 1998, he presented with multiple, firm, shiny, asymptomatic nodules on the back and abdomen. A skin smear from one of the nodules showed a BI of 6+; his mean BI was 4+. A biopsy from a nodule on the abdomen was reported as nodular lepromatous leprosy with histoid areas.
Although the mouse foot pad study showed viable bacilli in T900r mice, the drug sensitivity tests were inconclusive since both the drug group and the control group of the normal CBA mice did not show multiplication of AFB.
He was started on WHO/MDT. At the end of 1 year of treatment the nodules showed regression and his BI came down to 3.75+.
Case No. 3. A 42-year-old female was seen in 1973 with diffuse skin infiltration all over the body and bilateral thickening of the ulnar nerves. Her skin smears from the routine sites showed a mean BI of 3.5+. She was diagnosed as lepromatous leprosy and started on dapsone monotherapy. After 5 years of treatment with dapsone her skin smears were negative for AFB. She was continued on dapsone monotherapy, which she had for a total period of 9 years. In July 1982 she was included in the THELEP- sponsored field trials of MDT. At the time of induction into the trial she was clinically inactive, her skin smears were negative for AFB, and she was lepromin negative. She received MDT consisting of monthly supervised doses of 600 mg rifampin and 600 mg clofazimine on two consecutive days a month, bimonthly injections of 225 mg acedapsone, and a daily dosage of 100 mg of dapsone unsupervised. After 2 years she was released from treatment. During the yearly follow up she was clinically inactive and her skin smears from routine sites were negative for AFB.
Fourteen years after completion of MDT, in November 1998, she reported with multiple firm, shiny, nontender nodules in groups on her upper limbs and back. Her mean BI was 3.25+. A biopsy from a nodule on the right arm was consistent with nodular lepromatous leprosy with histoid areas.
Although the mouse foot pad study showed viable bacilli in T900r mice, the drug susceptible tests were inconclusive since both the drug group and the control group of the normal CBA mice did not show multiplication of AFB.
She was started on WHO/MDT, but was highly irregular in treatment. One year after starting treatment she had received only five pulses of MDT. At the end of 1 year the nodules were still present and she was clinically active, although her mean BI had fallen to 2.83+.
DISCUSSION
Histoid leprosy occurs in lepromatous leprosy patients who relapse after receiving treatment with dapsone monotherapy for many years (1,5,13). In 1982 the World Health Organization (WHO) introduced multidrug therapy (WHO/MDT), consisting of dapsone, rifampin and clofazimine for the treatment of MB leprosy patients (7). MDT has shortened the duration of treatment, brought down the prevalence of leprosy drastically, and has a very low relapse rate (<1%) (l4).
Our patients received adequate doses of MDT containing dapsone, clofazimine and rifampin for a period of 2 years. They were repeatedly found to be clinically inactive and their skin smears were negative during the yearly follow up. Twelve to 15 years after completion of treatment with MDT they relapsed as histoid leprosy. We report these cases because they relapsed as histoid leprosy after taking MDT consisting of dapsone, rifampin and clofazimine for 2 years. Relapse as histoid leprosy, which was seen in the dapsone monotherapy era, was not reported in post-MDT relapses until recently. To the best of our knowledge only one case of leprosy relapsing as histoid after receiving MDT has been reported so far (3).
Two THELEP-supported field trials have been conducted at SLR&TC, Karigiri. In the first trial, 1067 B1 and LL patients were inducted between December 1981 and December 1982 and were treated with WHO- recommended MDT regimens for a minimum period of 2 years or until skin-smear negativity, whichever occurred later. No relapse was observed in any patient until the end of July 1995 after a total duration of follow up of 8244 person-years after RFT (4). Most of the patients had had long-term dapsone monotherapy prior to inclusion in the trial. Only 44 were newly detected and had had no previous therapy for leprosy. Of these 44 patients, 34 (3 had died and 7 had migrated) could be re-evaluated 17 years after induction into the trial. No relapse was seen by the end of December 1999 after a mean duration of follow up of 13.7 ± 1.38 years and a total duration of follow up of 466 person-years (submitted for publication).
In the second trial, 360 newly diagnosed and previously untreated BL and LL patients were included from 1984 to 1994 and were treated with the same regimens for a fixed duration of 2 years (FDT). Until the end of July 1995, after a total duration of follow up of 886 person-years after RFT, no relapse was seen in this trial also (12). In this trial, 65 patients had a mean BI of 3+ or more, of which 57 had successfully completed the treatment. Of these 57 patients, 46 with a mean BI of 3.79 ± 0.55+ were reevaluated (5 had died and 6 had migrated). By the end of March 2000 after a total duration of follow up of 424 person-years and a mean duration of follow up of 9.3 ± 2.98 years, only one relapse was seen, giving a rate of 2.17% or a risk of 0.23 per 100 person-years in patients with a BI of >3+ (submitted for publication).
Relapse in our patients could have been caused by persisters or re-infection with exogenous Mycobacterium leprae. Persisters are bacilli that adapt to adverse conditions in their environment by reducing their metabolism to a minimum. They are capable of surviving in their host despite adequate chemotherapy. They may remain in this dormant state for some time; a proportion of them may die, but some bacilli may regain normal metabolism and start multiplying, causing relapse. The duration of this dormant stage is not known; it may be a few months to a few years (11).
In leprosy, more so in BL and LL patients, there is impaired host cellular immune response to M. leprae (l0); a treated case of leprosy remains susceptible to re-infection with exogenous M. leprae after RFT. The risk is more prominent in areas where leprosy is endemic.
Since our patients, treated cases of BL and LL leprosy belonging to a leprosy-endemic area, relapsed 12 to 15 years after RFT, the exact cause of relapse-persisters or re-infection with dapsone-resistant bacilli-could be anybody's guess.
Acknowledgment. We are thankful to Professor Charles K. Job, Consultant Pathologist, St. Thomas Hospital and Leprosy Center, Chettupattu; Dr. P. S. S. Sundar Rao, Director, SLR&TC, Karigiri and Dr. S. Arunthathi, Head, Department of Medicine, SLR&TC, Karigiri, for their encouragement and guidance.
REFERENCES
1. Chaudhury, D. S., Chaudhury, M. and Armah, K. Histoid variety of lepromatous leprosy. Lepr. Rev. 42(1971)203-207.
2. Desikan, K. V. and Iyer, C. G. S. Histoid variety of lepromatous leprosy. Int. J. Lepr. 40(1972)149-156.
3. Ebenezer, G. J., Barkatakj, A. and Job, C. K. Leprosy relapse presenting in a histoid form after multidrug therapy. Br. J. Dermatol. 140(1999)759-760.
4. Jesudasan, K., Vhayakumaran, P., Manimozhi, N., Sundar Rao, P. S. S. and Samuel, P. Effectiveness of MDT in multibacillary leprosy. Int. J. Lepr. 64(1996)128-132.
5. Mansfield, R. D. Histoid leprosy. Arch. Pathol. 41(1969)293-297.
6. Pfaltzgraff, R. E. and Ramu, G. Clinical leprosy. In: Leprosy. 2nd edn. Hastings, R, C., ed. Edinburgh: Churchill Livingstone, 1994, pp. 237-287.
7. Price, E. W. and Fitzherbert, H. Histoid lepromatous leprosy. Int. J. Lepr. 34(1966)367-374.
8. Ramos-Caro, F. A. Histoid lepromas. Cutis 30(1982)108-109.
9. Rodriguez, J. N. The histoid leproma: its characteristics and significance. Int. J. Lepr. 37(1969)1-21.
10. Ridley, D. S. and Jopling, W. H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966)255-273.
11. Toman, K. Bacterial persistence in leprosy. Int. J. Lepr. 49(1981)205-217.
12. Vhayakumaran, P., Jesudasan, K. and Manimozi, N. Fixed duration therapy (FDT) in multibacillary leprosy: efficacy and complications. Int. J. Lepr. 64(1996)123-127.
13. Wade, H. W. The histoid lepromas. (Abstract). Int. J. Lepr. 28(1960)469.
14. World Health Organization. The Leprosy Unit, Division of Tropical Diseases. Risk of relapse in leprosy. Geneva: World Health Organization, 1994. WHO/CTD/LEP/94.1.
1. M.B.B.S., M.D., Specialist, Department of Community Health.
2. M.B.B.S.,M.D., Head, Department of Histopathology and Experimental Pathology.
3. M.B.B.S., M.D., D.N.B., Head. Medical Officer, Department of Community Health, Schieffelin Leprosy Research and Training Center, Karigiri, Vellore District, Tamil Nadu, South India 632 106.
4. M.B.B.S., D.H.E., Specialist. Medical Officer, Department of Community Health, Schieffelin Leprosy Research and Training Center, Karigiri, Vellore District, Tamil Nadu, South India 632 106.
5. M.B.B.S., Medical Officer, Department of Community Health, Schieffelin Leprosy Research and Training Center, Karigiri, Vellore District, Tamil Nadu, South India 632 106.
Received for publication on 10 July 2000.
Accepted for publication in revised form on 30 August 2000.