- Volume 68 , Number 3
- Page: 277–82
Serious side effects of rifampin on the course of WHO/MDT: a case report
ABSTRACT
A male born in 1935 was diagnosed as having lepromatous leprosy when he was 17 years old. In addition to dapsone (DDS) monotherapy, he had been treated with rifampin (RMP) for 2 terms: first with 450 mg a day for 2 years when he was 39 years old; second with 150 mg a day for 2 months after a 1-year interval from the first regimen. During these entire courses with RMP, no complication was noted. When he was 64 years old in 1999, a diagnosis of relapsed borderline tuberculoid (BT) leprosy was made, and he was started on the multibacillary (MB) regimen of the World Health Organization multidrug therapy (WHO/MDT). After the third dose of monthly RMP, he developed a flu-like syndrome and went into shock. A few hours later, intravascular hemolysis occurred followed by acute renal failure. He was placed on hemodialysis for 7 series and recovered almost completely about 2 months later. The immune complexes with anti-RMP antibody followed by complement binding may have accounted for these symptoms. Twenty-four reported cases of leprosy who had developed side effects of RMP under an intermittent regimen were analyzed; 9 of the cases had had prior treatment with RMP but 15 had not. Adverse effects were more likely to occur in MB cases and were more frequent during the first 6 doses of intermittent regimens. The cases with prior treatment with RMP had had a higher incidence of serious complications such as marked hypotension, hemolysis and acute renal failure. However, many exceptions were also found, and we could not verify any fully dependable factor(s) to predict the side effects of RMP. More field investigation is desirable, and monthly administration of RMP must be conducted under direct observation through the course of WHO/MDT.RÉSUMÉ
Un homme ne.cn 1935 fut diagnostiqué atteint de lèpre lépromateuse à l'âge de 17 ans. En plus de la mono thérapie à la Dapsone (DDS), il a été traité par 2 fois à l'aide de Rifampicine (RMP); d'abord avec 450 mg par jour pendant 2 ans à l'âge de 39 ans, puis avec 150 mg par jour pendant 2 mois un an après le premier traitement. Durant les traitements, aucune complication ne fut détectée. A l'âge de 64 ans en 1999, un diagnostic de rechute de lèpre de type tuberculoide borderline (BT) fut posé, et le patient fut placé sous poly chimiothérapie traitant la lèpre multibacillaire (NB) préconisée par l'Organisation Mondiale de la Santé (PCT/OMS). Après la troisième dose mensuelle, il développa un syndrome grippal qui évolua rapidement vers le choc. Quelques heures après, une hémolyse intra-vasculaire apparut, suivie d'une insuffisance rénale aiguë. Il fut placé sous 7 séries d'hémodialyse et récupéra presque entièrement de cette complication environ 2 mois plus tard. Ces symptômes furent peut-être provoqués par des complexes immuns secondaires à des anticorps antiRMP fixant le complément. Vingt quatre cas rapportés de patients lépreux qui ont développés des effets secondaires liés à la RMP sous traitement intermittents ont été analysés; 9 de ces cas présentaient des commémoratifs de traitements antérieurs avec la RMP, les 15 autres aucun commémoratifs de traitements antérieurs à la RMP. Le risque de complications était plus élevé chez les patient MB et ces complications étaient plus fréquemment recontrées lors des 6 premières doses des administrations intermittentes. Les cas ayant eu des traitements antérieurs à la RMP ont présenté une incidence plus élevée de complications sérieuses telles des hypotensions sévères, des hémolyses et des insuffisances rénales aiguës. Cependant, de nombreuses exceptions furent mises en évidence et aucun facteur prédictif net ne fut mis en évidence. Plus de recherche sur le terrain est indiqué, et l'adminstration mensuelle de RMP doit être conduite sous surveillance médicale stricte pendant toute la durée du traitement.RESUMEN
A un paciente masculino nacido en 1935 se le diagnostic© lepra cuando tenia 17 anos de edad. Ademas de monoterapia con dapsona el paciente recibio dos ciclos de de rifampina (RMP), primera con 450 mg diários por 2 anos cuando tenía 39 anos, y después con 150 mg diários por dos meses después de 1 ano de terminado el primer ciclo de tratamiento. Durante los do ciclos de tratamiento con RMP no se observo ninguna complicación. En 1999, cuando tenía 64 anos, se le diagnostico lepra tuberculoide subpolar (BT) y se comenzó a tratar con ía poliquimioterapia recomendada por la Organización Mundial de la Salud (OMS). Después de la tercer dosis mensual de RMP el paciente desarrolló un síndrome parecido a la influenza y entró en shock. Horas más tarde presentó hemólisis intravascular seguida pro falia renal aguda. Se sujetó a 7 series de hemodiálisis y se recupero casi completamente dos meses después. Estos sintomas pudieron haberse debido a la formación de complejos inmunes con anticuerpos hacia la RMP y a la activación dei complemento. Con este antecedente, se analizaron 24 casos de lepra que habían desarrollado efectos colaterales de la RMP administrada en ciclos intermitentes; 9 de los casos habían tenido un tratamiento prévio con RMP pero 15 pacientes no. Los efectos adversos fueron más frecuentes en los casos MB, y durante las primeras 6 dosis en los tratamientos intermitentes. Los casos con tratamiento prévio con RMP tuvieron mayor incidência de complicaciones serias tales como hipotensión, hemólisis y falia renal. Sin embargo, también se encontrarem muchas excepciones, de manera que no se pudo identificar ningún factor de predicción de los efectos colaterales de la RMP. Se requiere más trabajo de campo, y se recomienda que la administración mensual de la RMP se haga bajo observación directa mientras se administra la poliquimioterapia recomendada por la OMS.We recently came across a case of leprosy who developed serious complications due to rifampin (RFP) during the course of World Health Organization-recommended multiple drug therapy (WHO/MDT). Although the incidence of life-threatening side effects of RFP is believed to be very rare (7,24), we do not have enough information about it. Through our experience and reported documents, we present here the possible side effects of RFP aiming at more patient-oriented leprosy control.
CASE REPORT
A male born in 1935 was diagnosed as lepromatous leprosy (LL) when he was 17 years old. He had received dapsone (DDS) monotherapy for several years, and then his disease became quiescent. At the age of 29 (1964), reactivated skin lesions were found and streptomycin, kanamycin, thiambutosine, and DDS were given in a variety of combinations for 5 years. At the age of 39 (1974), his disease had not achieved complete cure, hence rifampin (RFP) 450 mg a day was given for 2 years. After a 1-year interval without chemotherapy, when he was 42 years old (1977), RFP 150 mg a day was administered for 2 months. During all this chemotherapy, no adverse effect was reported.
In November 1999, when he was 64 years old, he presented with skin lesions that he said had continued for more than 5 years. On examination, an infiltrated, anesthetic, erythematous lesion of about 28 x 10 cm having a well-defined margin was noticed on his left flank. A few, red pea-sized papules were also noticed around the large lesion. Neither thickening nor tenderness was found on his peripheral nerves. All skin smears taken from several sites were negative. A skin biopsy was taken from the infiltrated skin lesion. From the clinico-pathological findings, the diagnosis of borderline tuberculoid (BT) relapse was made.
He was started on WHO/MDT for multibacillary (MB) leprosy (2,5). The first and second monthly doses of RFP and clofazimine were taken under direct observation without any problems. The third dose was taken in January of 2000 while the patient was traveling. Fifteen minutes after he took the third monthly dose of RFP, abdominal pain, myalgia, fever with chills and vomiting developed. Remarkable swelling and redness were noticed on his face by the attending physician before he went into shock. His systolic blood pressure (BP) was approximately 50 mm Hg. Soon after intravenous fluid administration containing furosemide and corticosteroid, he was referred to a nearby hospital. Approximately 2 hr later when he arrived at the hospital, his BP was 129/102 with a body temperature of 39.1°C. On the afternoon of the same day, his general condition improved, having normal body temperature, but blood-colored urine and apparent oliguria of about 100 ml in a half day were noticed. On the next day, hemodialysis (HD) therapy was initiated with a diagnosis of disseminated intravascular coagulation and an antibiotic (minocycline) was given for the fear of unknown infection. On the second day, high fever was noticed and bacterial infection through the intravenous line was suspected. Minocycline was replaced with meropenem trihydrate for several days. On and after the third day, no fever was noticed. On the third day, a transient tar-like stool was found and erosive gastritis was found by gastrointestinal fiber. Colon fiber could not be done without patient's consent. Two weeks later, after 7 series of hemodialysis the patient got into the diuretic phase and hemodialysis was discontinued.
LABORATORY FINDINGS
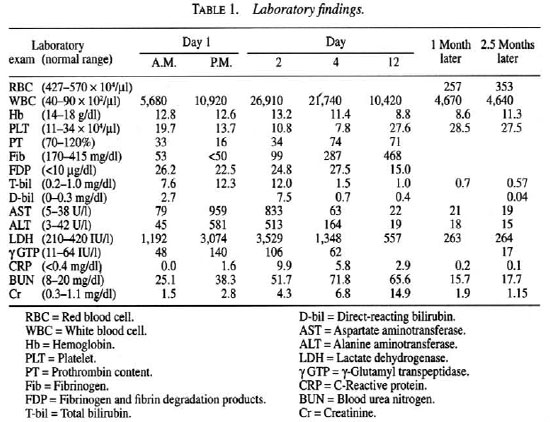
Laboratory reports kindly offered by Dr. Fukushima in the referral hospital are shown in Table 1 according to the time lapse. The most prominent results were found in the blood clotting system. The prothrombin content (PT), fibrinogen (Fib), Fib and fibrin degradation products (FDP) were very low from the inception, accompanied by high levels of total and direct-reacting bilirubin (T-bil, D-bil), aspartate aminotransferase (AST), alanine aminotransferase (ALT), and lactate dehydrogenase (LDH). The blood urea nitrogen (BUN) and creatinine (Cr) levels gradually increased and their maximum were seen on the fourth and twelfth days, respectively. Throughout this period, thrombocytopenia could not be seen. One month later, the blood chemistry data fairly improved, although the Cr titer was slightly high accompanied with anemia. About 2.5 months later, the creatinine level became normal but mild anemia was still noticed.
DISCUSSION
The clinical feature just after the third dose of RFP indicates a flu-like syndrome and subsequent anaphylactic shock. Two hours later, the patient's general condition had recovered but intravascular hemolysis was noticed (Table 1). Based on the low ratio of D-bil to T-bil, the high levels of AST, ALT and LDH are considered to be caused by hemolysis, although transient RFP-induced hepatitis cannot be ruled out with certainty. Between the second and fourth day, hemolysis began to recover, as shown in the rapid improvement of the related data. On the other hand, abnormal renal function was prolonged further, and it took more than 2 months to reach near complete recovery. The interference of suspected infection and the antibiotics used are uncertain.
Eventually, flu-like syndrome, anaphylactic shock, intravascular hemolysis and acute renal failure (ARF) developed in our case, and all these symptoms had not been noticed 23 years ago during daily administration of RFP in a smaller dosage.
Pathogenesis. Although the pathogenesis of flu-like syndrome has not been clearly identified, many studies suggest hypersensitivity reaction due to circulating immune complexes (11,14,17). In our case, it might also be explained that this symptom was the prodrome of subsequently occurring intravascular hemolysis.
While a Type I hypersensitivity reaction might be the most understandable pathogenesis for an anaphylactic reaction, an immune complex-mediated reaction can also cause similar symptoms through the overproduction of inflammatory cytokines (11).
The rapid onset of hemolysis suggests that the third dose of RFP in our case must have bound to the pre-existing circulating anti-RFP antibodies forming immune complexes. Then they might have bound on the surfaces of blood cells and destroyed them by complement fixation (10,17,22).
As the cause of ARF, tubular necrosis resulted from systemic hypovolemia (anaphylaxis) could be mentioned. However, the most likely pathogenesis in our case is the interstitial nephritis with renal tubular necrosis caused by the adhesion of immune complexes and complement binding (4,10,11,17,22).
For all the symptoms which occurred in our case, the immune complexes or Type III hypersensitivity reaction is suspected to have the main role through its adhesion to antigens presented on the surface of blood cells, endothelial cells, renal tubular epithelium and cytokine-producing cells (4). However, the exact pathogenesis cannot be proved from our limited data.
Frequency and risk factors. Flu-like syndrome, anaphylactic shock, intravascular hemolysis and ARF are known adverse reactions to RFP (5); however, the incidence in leprosy patients is indicated to be very low (7,24).
In the report about complications of WHO/MDT in Brazil (3), the incidences of flu-like syndrome, ARF, hemolytic anemia, and hypotension are calculated as 0.3%, 0.1%, 0.03%, and 0.01%, respectively. Although both RFP and DDS should be taken into consideration in most of these symptoms, we are not quite sure whether the incidence of ARF (0.1%), for example, is low enough to be declared as a "very rare side effect of RFP." In this report, they suggested the progressive increase in the incidence of flu-like syndrome according to senility and predominant complications among MB cases having prior treatment with DDS and RFP.
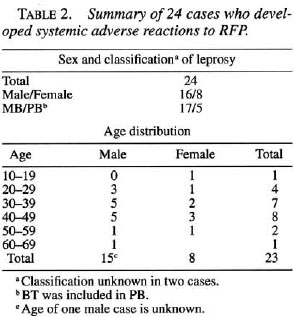
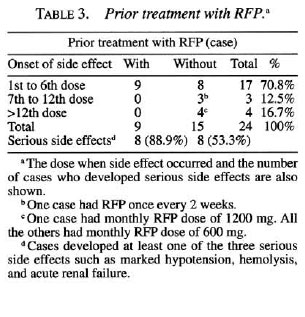
In other reports which we were kindly informed of by Dr. V. K. Pannikar, Steering Committee on Chemotherapy of Leprosy, WHO, we found 24 leprosy cases who had developed at least one of the four symptoms which occurred in our case under intermittent administration of RFP (Table 2) (1,6,8,12,13, 15, 16, 18-23) prom these reports, predominant incidence in MB cases (Table 2) and increasing tendency with aging (Table 2) are suspected, compatible with the report from Brazil (3). The longer period of treatment in MB cases and the predominance of humoral immunity in MB and/or older cases might account for these results. However, there are many exceptions. Whether they had prior treatment with RFP (daily or intermittent) before the start of intermittent regimen is shown in Table 3, along with the doses when complications occurred and the number of cases who developed at least one of the three serious side effects such as marked hypotension (anaphylactic), hemolysis and ARE Out of 24 cases, 9 had had previous RFP followed with various intervening periods without RFP. Fifteen cases had had no previous RFP. The adverse effects were likely to occur during the first six doses, especially in cases having had previous RFP. On the other hand, four cases (16.7%) without previous RFP developed adverse effects after more than the twelfth dose. The serious side effects occurred in 8 cases (88.9%) out of 9 with previous RFP and 8 cases (53.3%) out of 15 without previous RFP, indicating that cases who have had previous RFP are more likely to develop serious complications than cases not previously treated with RFP. Fortunately, most of these cases have recovered completely.
Our case had previously taken RFP and had developed serious complications on the third monthly dose of RFP. No events had been noticed 23 years ago when he had restarted daily RFP at a smaller dosage after a 1-year interval. The overproduction of antibody or insufficient clearance of immune complexes caused by the monthly administration (9'14), along with the senile change of his immunological status, might have contributed to the occurrence of his episode. There is another possibility that desensitization or immune tolerance might have accounted for the absence of adverse reaction during his daily regimen, although the mechanism has not been clarified enough (2).
Now we adhere strictly to the principle that the monthly administration of RFP must be conducted under direct observation during the entire course of WHO/MDT.
We can enumerate some factors that may predispose to complications of an intermittent regimen with RFP; however, to our knowledge, there is no completely reliable predictive factor. More information based on enough case holding in a larger cohort is desirable, along with the strict supervision of each monthly administration of RFP.
Acknowledgment. We wish to express our gratitude to Dr. Miyagi, Director General, National Sanatorium Amami-Wakoen; Dr. M. Fukushima, Kagoshima Prefectural Hospital; and Dr. Shimada, National Sanatorium Tama-Zenshoen for their appropriate treatment and sincere support offered to the patient in addition to their kind cooperation on this report. We also thank many executive staff members of Japanese pharmaceutical companies and Dr. M. Gidoh, National Institute for Leprosy Research in Tokyo, for their efforts and assistance in this report. We gratefully acknowledge Dr. V. K. Pannikar, Steering Committee on Chemotherapy of Leprosy, WHO. This report was accomplished with his kind information about the literature relating to the complications of RFP.
REFERENCES
1. Al-Samie, A. R. and Al-Qubati Y. "Flu" syndrome on monthly rifampin dose; first case reported from Yemen. Int. J. Lepr, 63(1995) 574-576.
2. Bell, I. J. and O'Hehir, R. E. Immune mechanisms of disease. In; Oxford Textbook of Medicine. 3rd edn. Weatherall, D. J., Ledingham, J. G. G. and Warrell, D. A., eds. Oxford: Oxford University Press, 1996, pp. 162-163.
3. Brasil, M. T. L. R. F., Opromolla, D. V. A., Marzliak, M. C. L. and Nogueira W. Results of a surveillance system for adverse effects in leprosy's WHO/MDT, int. J. Lepr. 64(1996)97-104.
4. De Vriese A. S., Robbrecht, D. L., Vanholder, R. C., Vogelaerse, D. P. and Lameire, N. H. Rifampin-associated acute renal failure; pathophysiologic, immunologic, and clinical features. Am. J. Kidney Dis. 31(1998)108-115.
5. Girling, D. J. and Hitze, K. L. Adverse reactions to rifampicin. Bull. WHO 57( 1979)45-49.
6. Gupta, A., Sakhuja, V., Gupta, K. L. and Chugh, K. S. Intravascular hemolysis and acute renal fail ure following intermittent rifampin therapy. Int. J. Lepr. 60(1992)185-188.
7. Jopling, W. H. Side effects of antileprosy drugs in common use. Lepr. Rev. 54(1983)261-270.
8. Kar, H, K. and Roy, R. G. Reversible acute renal failure due to monthly administration of rifampicin in an leprosy patient. Indian J. Lepr. 56(1984)835-839.
9. Kleinknecht, D., Homberg, J. C. and Df.croix G. Acute renal failure after rifampicin. Lancet 1(1972)1238-1239.
10. Lakshminarayan, S., Sahn, S. A. and Hudson, L. D. Massive haemolysis caused by rifampicin. Br. Med. J. 2(1973)282-283.
11. Martinez, E., Collazos, J. and Mayo, J. Hypersensitivity reactions to rifampin; pathogenic mechanisms, clinical manifestations, management strategies, and review of the anaphylactic-like reactions. Medicine 78(1999)361-369.
12. Naafs, B. and Matemera, B. O. A possible "flu" syndrome on once-monthly rifampicin. Lepr. Rev. 57(1986)271-273.
13. Nishioka, S. A., Goulart, I. M. B., Araujo, F. R. F. N., Arantes, S. C. F., Burgarelli, M. K. N., Ferreira, M. S., Santos, R. R and Lopes, V. R. Severe thrombocytopenia and intermittent use of rifampin. Int. J. Lepr. 60(1992)273-274.
14. O'Mahony, M. G. and Kar, C. W. Relationship between rifampicin-dependent antibody scores, serum rifampicin concentrations and symptoms in patients with adverse reactions to intermittent rifampicin treatment. Clin. Allergy 3(1973)353-362.
15. Parking, A. A. and Shah, B. H. Flu like syndrome with rifampicin pulse therapy. Indian J. Lepr. 61(1989)209-211.
16. Patki, A. H., Jadhav, V. H. and Mehta, J. M. "Flu" syndrome on once monthly rifampicin. Indian J. Lepr. 60(1988)84-86.
17. Pujet, J. C., Homberq, J. C. and DtCROix, G. Sensitivity to rifampicin; incidence, mechanism, and prevention. Br. Med. J. 2(1974)415-418.
18. Rajan, M. A., Soundararajan, R., Krishnamurthy, V. and Ramu, G. Acute renal failure following rifampicin. Indian J. Lepr. 59(1987)286-291.
19. Ramachandran, A. and Bhatia V. N. Rifampicin induced shock; a case report. Indian J. Lepr. 62(1990)228-229.
20. Sule, R. R. An unusual reaction to rifampicin in a once monthly dose. Lepr. Rev. 67(1996)227-233.
21. Thomas, A., Joseph, P. and Prabhakar, R. "Flu" syndrome associated with other systemic manifestations with once a month rifampicin in the treatment of multibacillary leprosy. Indian J. Lepr. 65(1993)219-224.
22. Tomonaga, H. Detection of antibody specific to rifampicin metabolite by ELISA; mechanism of sensitization by rifampicin. Arerugi 42(1993)854-863.
23. Vaz, M., Jacob, A. J. W. and Rajendran, A. "Flu" syndrome on once monthly rifampicin; a case report. Lepr. Rev. 60(1989)300-302.
24. WHO Study Group. Chemotherapy of leprosy. Geneva: World Health Organization, 1994, p. 7. Tech. Rep. Ser. 847.
25. World Health Organization. Chemotherapy of leprosy for control programmes. Geneva: World Health Organization, 1982. Tech, Rep. Ser. 675.
1. M. Namisato, M.D., Director of Dermatology, National Sanatorium Tama-Zenshoen, 4-1-1 Aobacho, Higashimurayamashi, Tokyo 189-8550, Japan.
2. M.D., Professor, Department of Dermatology, Juntendo University School of Medicine, 2-1-1 Hongoh Bunkyoku, Tokyo 113-8421, Japan.
Reprint requests to Dr. Namisato at the above address or FAX 81-42-394-2410; email: makonami@pop2l.odn.ne.jp
Received for publication on 5 May 2000.
Accepted for publication on 1 August 2000.