- Volume 68 , Number 3
- Page: 323–5
Dapsone drug resistance in the MDT era
To the Editor:
The specter of drug-resistant leprosy has receded with the advent of multidrug therapy (MDT). However, there are few laboratories remaining which have the facility to measure drug resistance by the mouse foot pad method. Hence, it has been difficult to ascertain what the current state of drug resistance is and what the trends in resistance patterns after MDT implementation have been.
In Nepal, dapsone (DDS) monotherapy was introduced in 1956 and MDT in 1983, although the coverage of MDT only reached 95% more than a decade later. Since 1987, we have been testing all suitable patients for primary and secondary drug resistance using the mouse foot pad model (3). We have analyzed the drug sensitivities of Mycobacterium leprae isolated from skin biopsies from 268 new and relapsed cases and from patients re-starting treatment (The Table). In the period 1988 to 1999, there was a decline in the prevalence of acquired dapsone resistance and a rise in the prevalence of primary dapsone resistance (The Figure). There was a complete absence of rifampin (RMP) resistance among our patients.
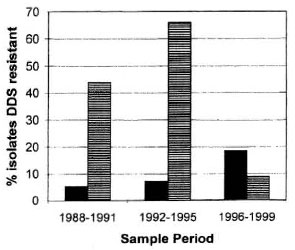
The Figure. Prevalence of dapsone resistance in Nepal, 1988-1999.  = Primary resistance; = acquired resistance.
= Primary resistance; = acquired resistance.
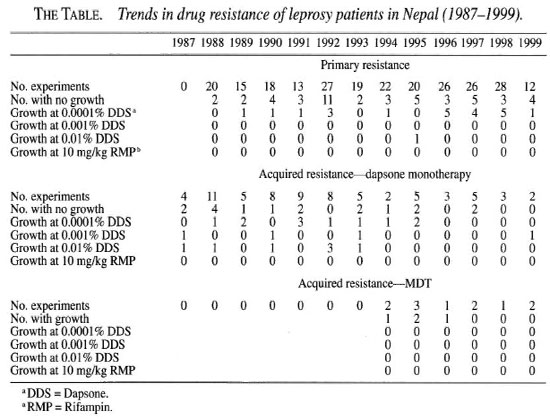
Primary dapsone resistance arises by the infection of new persons with resistant bacteria often shed by a person with acquired resistance to dapsone. During the late 1960s and 1970s, there were alarming reports of increasing secondary and primary dapsone resistance at the end of the dapsone monotherapy era, which prompted the introduction of MDT (2, 4). From our data it appears that secondary resistance to dapsone does not develop under MDT. Hence, the decline in secondary resistance is due to the introduction of MDT in Nepal and to the cure of secondary dapsone-resistant cases with MDT. By contrast there was a significant increase in the prevalence of primary dapsone resistance in new, previously untreated cases (trend in proportion test χ = 4.98, p <0.05, The Table). All except one isolate was resistant to low-level dapsone (0.1 mg) and thus sensitive to doses of dapsone used in MDT. Hence, the clinical response to MDT in these patients will not be compromised. During the monotherapy era the level of dapsone resistance increased step-wise and resistance at high levels became common (4). It will be important to monitor trends in the level of resistance as well as the frequency of primary dapsone resistance in the future. MDT efficacy could be severely compromised if high-level primary dapsone resistance becomes highly prevalent.
The genetic basis of dapsone resistance has recently been described (5). Although genetic detection of drug resistance is technically sophisticated, the application of the technology in selected leprosy-endemic areas will allow a more rapid and widespread assessment of the problem. The gene method must first be validated by paralleled mouse foot pad experiments in order to be sure what level of dapsone resistance is being detected. Although dapsone has a weaker bactericidal action than rifampin, we need to prevent the development of resistance to any component drug in MDT schedules. One could imagine a dangerous scenario developing in which high level primary dapsone resistance and poor clofazimine compliance leads effectively to rifampin monotherapy, a condition in which it is already known that rifampin resistance in leprosy can develop (1).
One of the unintentional but distressing effects of the leprosy elimination program has been the decline in mouse foot pad laboratories around the world. This has led to the situation where an elimination campaign based entirely on drug therapy has little or no measure of the extent of and trends in drug resistance. Our data show some important trends and should encourage the remaining mouse foot pad laboratories in the world to continue their important work of assessing drug resistance in leprosy.
Acknowledgment. The Mycobacterial Research Laboratory is supported by The Leprosy Mission International. The authors gratefully acknowledge the assistance of the staff of the mouse foot pad laboratory.
- Paul W. Roche, Ph.D. Kapil Dev Neupane
Sarah S. Failbus, M.Sc.
C. Ruth Butlin, M.B.B.S.
Mycobacterial Research Laboratory
Anandaban Leprosy Hospital
P.O. Box 151
Kathmandu, Nepal
Fax: 977-1-290-538,
email: anandaban@mail.com.np
REFERENCES
1. Grosset, J. H., Guelpa-Lauras, C.-C., Bobin, P., Brucker, G., Cartel, J.-L., Constant-Desportes, ML, Flaguel, B., Frederic, M., Guillaume, J.-C. and Millan, J. Study of 39 documented relapses of MB leprosy after treatment with rifampin. Int. J. Lepr. 57(1989)607-614.
2. Pearson, J. M. H., Haile, G. S. and Rees, R. J. Primary dapsone resistant leprosy. Lepr. Rev. 48(1977)129-132.
3. Shepard, C. C. and Chang, Y. t. Effect of several antileprosy drugs on the multiplication of the human leprosy bacillus in foot pads of mice. Proc. Soc. Exp. Biol. Med. 109(1963)636-638.
4. WHO Expert Committee on Leprosy. Fifth report. Geneva: World Health Organization, 1977. Tech. Rep. Ser. 607.
5. Williams, D. L., Spring, L., Harris, E., Roche, P. and Gillis, T. P. Dihydropteroate synthase of Mycobacterium leprae and dapsone resistance. Antimicrob. Agents Chemother. 44(2000)1530-1537.