- Volume 68 , Number 3
- Page: 325–7
Tumor necrosis factor promoter polymorphism (TNF2) seems to protect against development of severe forms of leprosy in a pilot study in brazilian patients
To the Editor:
Leprosy is a chronic infectious disease characterized by clinical forms which are associated to the immune response developed by the host against the bacteria. Polar forms are either paucibacillary (PB; tuberculoid leprosy) with a pronounced cell-mediated immune response (CMI) or multibacillary (MB; lepromatous leprosy), which lacks CMI. Believed to be an important protection mediator against infections, tumor necrosis factor-alpha (TNF-α), one of the key cytokines in CMI, is an inducible factor covering a wide range of proinflammatory and immunostimulatory activities (3). Moreover, depending on the quantity and the time period over which its production is sustained, TNF-α may exert a beneficial or a deleterious effect. For example, in leprosy, enhanced production of TNF-α has been associated with the development of such immunopathological states as nerve damage (10) and inflammatory reactional episodes (5) as well as with the development of the more benign tuberculoid form of the disease (12). High TNF-α levels were detected in the serum during reaction (11) and in vitro, following stimulation of the peripheral blood mononuclear cells, both in tuberculoid and reactional patients (1).
It has been suggested that development of a particular type of leprosy may be genetically determined (2) and could be responsible for the inter-individual differences in the immune response during the disease. Expression of TNF-α is tightly controlled at the transcriptional and post-transcriptional levels, and a particular single nucleotide polymorphism at the -308 position within the regulatory region of the TNF-α gene generates the allelic TNF2 form shown to be associated with enhanced TNF-α production (15) and to severe forms of some inflammatory and auto-immune diseases (7). In this connection, Roy, et al. (9) have recently described the significant association between TNF2 allele frequency and polar lepromatous-type leprosy in an Indian population.
In a study carried out in the Leprosy Laboratory at the Oswaldo Cruz Foundation, Rio de Janeiro, Brazil, TNF2 allele frequency was determined in 92 healthy control individuals and in 300 leprosy patients classified on the basis of their clinical and histologic features (s) as suffering from PB (BI negative patients, N 90; 2 BT, 63 TT, 15 pure neural form and 10 indeterminate) or MB leprosy (BI positive patients, N = 210; 85 BL, 70 LL, and 55 BB). Genomic DNA was prepared from frozen whole blood (300 µl) by a commercially available DNA extraction kit (Gibco BRL, Gaithersburg, Maryland, U.S.A.) and typing of the TNF-α promoter region (107 bp fragment) for analysis of polymorphisms at the -308 position was performed with use of specific primers through a single polymerase chain reaction (PCR) step and further digestion with Ncol (14). Comparison of genotype frequencies among the groups was determined by means of the chi-squared test.
The results presented as allelic frequencies are shown in The Table. Our present data indicate that the presence of TNF2 protects against the development of the more severe form(s) of leprosy. A significant (χ2 = 7.55, p = 0.005) higher frequency of the mutant allele was observed in the control group (16.3%) when compared to the leprosy patients as a whole (10.8%). In addition, the TNF2 frequency rates, while similar for the controls and the PB patients (14.4%), were significantly (χ2 = 9.63, p <0.005) higher than that observed for the MB group (9.3%). Nevertheless, the proportion of TNF2, albeit enhanced in the PB versus MB patients, was not statistically significant (χ2 = 2.48, p >0.05).
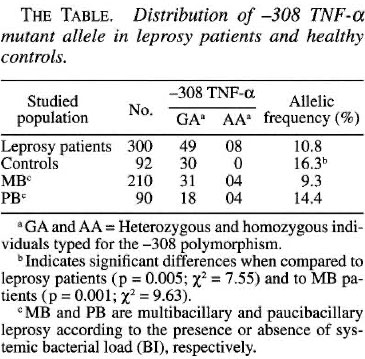
The significant association of TNF2 to the control group described herein supports the idea that the presence of TNF2 could be a predisposing genetic factor against development of the disease. This observation is in contrast to the findings of Roy, et al. (9), who reported a noteworthy association between MB leprosy and TNF2.
As is well known, the protective role of TNF-α in mycobacterial disease has been suggested in earlier studies by demonstrating that a) immunization against TNF-α blocked granuloma formation in mice in response to Mycobacterium bovis (BCG) infection (s); b) it reduced the survival time of animals following infection with virulent M. tuberculosis or Listeria monocytogenes (4); and c) TNF-α increased the resistance against M. avium infection (13).
Another difference between our study results and those of Roy, et al, is that the allele frequency values observed in the Brazilian control group were 5.5 times higher than those found among the Indian population which, while not due to a higher frequency rate of the homozygous TNF2 genotype, could be related to differences in the ethnic composition of each group associated with HLA haplotypes in each population. Rio de Janeiro is also highly endemic for leprosy with a prevalence rate of 4.4/ 10,000 (WHO, 1999). Nonetheless, whereas the Indian population is seen as being overall homogeneous, the Brazilian population is characterized by its mixed racial and ethnic components, which might at least partially explain the opposing results.
In conclusion, the data provided by our study strongly point to the TNF2 allele's protective role in defense against the development of leprosy. It is also important to mention that in future studies of this nature, the particular features of a certain population group must always be taken into account in study design and analysis.
Acknowledgment. We would like to thank A. M. Sales, D. M. Vieira and L. M. M. Vieira for clinical follow up on the patients and data information. A, S. Almeida is supported by a grant from CNPq, Brazil. This study was supported by the World Health Organization/World Bank/TDR ID 930222.
- Adalberto R. Santos, Ph.D.
Alexandre S. Almeida, Ms.C.
Leprosy Laboratory
- Philip N. Suffys, Ph.D.
Laboratory of Molecular Biology and
Diagnosis of Infectious Disease
- Milton O. Moraes, Ph.D.
Valcemir F. S. Filho
Leprosy Laboratory
Oswaldo Cruz Institute
FIOCRUZ
Rio de Janeiro, Brazil
- Haroldo J. Mattos, M.D., Ph.D.
Informatica Medica
Faculty of Medical Science
State University of Rio de Janeiro
Rio de Janeiro, Brazil
- Jose A. C. Nery, M.D., Ms.C.
Leprosy Laboratory
- Pedro H. Cabello, Ph.D.
Laboratory of Genetics
- Elizabeth P. Sampaio, M.D., Ph.D.
Euzenir N. Sarno, M.D., Ph.D.
Leprosy Laboratory
Oswaldo Cruz Institute
FIOCRUZ
Av. Brasil 4365, Manguinhos
21045-900 Rio de Janeiro, RJ, Brazil
REFERENCES
1. Barnes, P, F., Chatterjee, D., Brennan, P. J., Rea, T. H. and Modlin, R. L. Tumor necrosis factor production in patients with leprosy. Infect. Immun. 60(1992)1441-1446.
2. Eden, W. V. and de Vries, R. R. P. HLA and leprosy: a re-evaluation. Lepr. Rev. 55(1984)89-109.
3. Fiers, W. Biologic therapy with TNF: preclinical studies. In: Biology Therapy of Cancer, 2nd edn. De Vita, V. T., Jr., Hellman, S. and Rosenberg, S. A., eds. New York: J. P. Lippincott Co., 1995, pp. 295-327.
4. Flynn, J. L., Goldstein, M. M., Chan, J., Triebold, K. J., Pfeffer, K., Lowenstein, C. J., Schreiber, R., Mak, T, W. and Bloom, B. R. Tumor necrosis factor-α is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity 2(1995)561-572.
5. Khanolkar-Young, S., Rayment, N., Brickell, P. M., Katz, D. R., Vinayakumar, S., Colston, M. J. and Lockwood, D. N. J. Tumor necrosis factor-alpha(TNF-α)synthesis is associated with the skin and peripheral nerve pathology of leprosy reversal reactions. Clin. Exp. Immunol. 99(1995)196-202.
6. Kindler, V., Sappino, A. P., Grau, G. E., Piguet, P. F. and Vassali, P. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell 56(1989)731-740.
7. Mira, J. P., Cariou, A., Grall, F., Delvaux, A., Losser, M. R., Heshmati, F., Cheval, C., Monchi, M., Teboul, J. L., Riche, F., Leleu, G., Arbfbe, L., Mignon, A., Delpech, M. and Dhainaut, J. F. Association of TNF2, a TNF-α promoter polymorphism, with septic shock susceptibility and mortality; a multicenter study. JAMA 282(1999)561-568.
8. Ridley, D. S. and Jopling, W. H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966)255-273.
9. Roy, S., McGuire, W., Taylor, C. G. M., Saha, B., Hazra, S. K., Hill, A. V. and Kwiatkowski, D. Tumor necrosis factor promoter polymorphism and susceptibility to lepromatous leprosy. J. Infect. Dis. 176(1997)530-532.
10. Sampaio, E. P. and Sarno, E. N. Role of inflammatory cytokines in tissue injury in leprosy. Int. J. Lepr. 64(1996)S69-S74.
11. Sarno, E. N., Grau, G. E., Vieira, L. M. M. and Nery, J. A. C. Serum levels of tumor necrosis factoralpha and interleukin-1(3 during leprosy reactional states. Clin. Exp. Immunol. 84(1991)103-108.
12. Silva, C. L. and Foss, N. T. Tumor necrosis factor in leprosy patients. J. Infect. Dis. 159(1989)787-790.
13. Smith, D. A., Hansch, H. G. R., Bancroft, G. J. and Ehlers, S. T cell independent granuloma formation in response to Mycobacterium aviumrole of tumor necrosis factor-α and interferon-γ. Immunology 92(1997)413-121.
14. Wilson, A. G., de Vries, N., Pouciot, F. R., Di Giovine, F. S., van der Putte, L. B. A. and Duff, G. W. An allelic polymorphism within the human tumor necrosis factor-α; promoter region is strongly associated with HLAA1, B8 and DR3 alleles. J. Exp. Med. 177(1993)577-560.
15. Wilson, A. G., Symons, J. A., McDowell, T. L., Di Giovine, F. S. and Duff, G. W. Effects of a tumor necrosis factor(TNF-alpha)promoter base transition on transcriptional activity. Br, J. Rheumatol. 33(1994)89-92.
Reprint requests to Dr. Sarno at the above address or FAX 55-21-270-9997.