- Volume 68 , Number 3
- Page: 328–30
Single-dose treatment for single lesion leprosy; histopathological observations
To the Editor:
The World Health Organization (WHO) in 1997 recommended single-dose rifampin-ofloxacin-minocycline (ROM-1) as the therapy for single lesion paucibacillary (PB) leprosy and, subsequently, the National Leprosy Elimination Program in India adopted this and implemented it all over the country. The single-dose therapy has also been tried for two to five lesions of leprosy with satisfactory results (2, 4, 5, 7).
This short course (single-dose) chemotherapy has been implemented for the treatment of leprosy. Apart from its clinical efficacy, this strategy has the additional advantages of saving manpower and resources, and increasing patient compliance. The clinical response to ROM-1 therapy has been found to be as effective as PB-MDT for single lesion PB leprosy (6,10). The observations on feasibility of long-term follow up (2), clinical problems and management (5), and the field implication (7) suggest satisfactory results.
However, some of the problems encountered with ROM therapy in single lesion PB patients reported so far are: a) persistence of existing lesions; b) increase in the size of old lesion and c) appearance of new lesions. These problems are reported to be encountered in about 4% of patients. Of these, 1.3% of the cases responded to steroid therapy, but 1.5% showed no improvement. The delayed clearance of granuloma may be associated with such clinical problems. However, no histopathological study has been reported following ROM-1 therapy.
We report our histopathological observations on 26 patients with single patch leprosy treated with ROM therapy. All of the patients underwent pre-treatment as well as post-treatment skin biopsy at the end of 12-18 months. The hematoxylin and eosin (H&E) sections were studied for the following parameters: a) type of infiltrate (granulomatous/lymphocytic); b) granuloma index (GI), and c) presence of acid-fast bacilli (AFB). GI is the fraction of the dermis in a section occupied by the granuloma. The GI is observed under low power objective and expressed decimally, e.g., 1 indicates the whole of the dermis is occupied by the granuloma, 0.1 indicates that 1/10 is occupied by the granuloma (3,8).
The histopathological changes were termed "active" when there was dermal infiltrate of epithelioid granuloma and the GI was more than 0.1 in the dermal tissue with nerve inflammation. It was termed "resolving" when the GI was less than 0.1 and "inactive" when granuloma was absent and/or the lymphocytic infiltrate was approximately <5%.
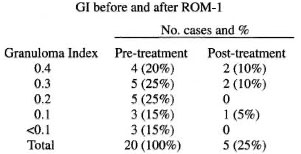
The above table shows a reduction in GI following ROM therapy. Out of 26 patients, 20 patients had granulomatous infiltrate (Fig. 1) and 6 patients showed perivascular, periappendageal and perineural lymphocytic infiltrate suggestive of indeterminate leprosy prior to initiation of therapy. These 20 patients were studied further for resolution of granuloma using the GI scale. At the end of the study, only 5 patients had granulomatous infiltrate, with total clearance of granuloma in the remaining 15 patients, indicating marked improvement following therapy (Fig. 2). Of these 5 patients with granulomatous infiltrate, 2 had a GI of <0.1, suggestive of resolving granuloma whereas only 3 patients had active granuloma at the end of the study. A striking resolution of granuloma was observed in patients with a high GI.
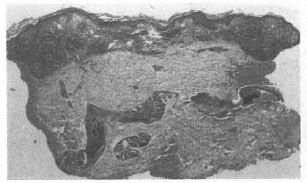
Fig. 1. Pretreatment photograph showing dense tuberculoid granulomatous infiltrate in dose apposition to thin epidermis before therapy (GI 0.4).
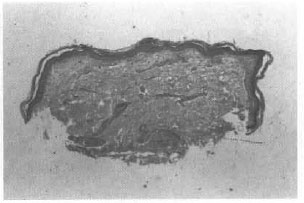
Fig. 2. Post-treatment photograph of the same patient showing complete resolution of granuloma 15 months after therapy (GI 0).
Histopathologically, signs of reaction were observed in one patient in the form of edema and extravasation of red blood cells. One patient with an indeterminate picture before therapy, showed granulomatous reaction (GI of 0.1) at the end of therapy. The remaining 5 patients with indeterminate histology had reached the inactive stage. One patient who developed a new lesion showed a reduction in GI in an old lesion (0.3 to 0.1) but the biopsy from the new patch showed a GI of 0.3. None of the patients showed the presence of AFB before or after therapy.
Although such studies are few, there are some reports of granuloma resolution following WHO/MDT. A histopathological study (7) of 30 patients treated with PBMDT for 6 months showed active granuloma in 4 patients at the end of 18-23 months. This provides a comparison with our current study with ROM-1 in which 5 patients showed histopathological activity at the end of 18 months.
In yet another investigation (1) in which clinical and histopathological activity were studied in 37 PB leprosy patients after 6 months of MDT, 8 patients showed histologically active persistent granuloma with significant reduction in GI at the end of 6 months. On follow up these patients showed continued regression of granuloma without further treatment (9). The histological improvement in 81% of the cases after 18 months following ROM-1 can be considered as satisfactory and is comparable to PB-MDT.
The results of these studies show that although histopathological improvement with reduction in granuloma size may take a longer time, eventually they all regress irrespective of which therapy (PB-MDT or ROM-1) is used. It was also observed that patients with a high GI show a faster resolution of granuloma, indicating the immunological basis of the granuloma formation and the resolution taking place perhaps once the bacterial antigen is eliminated.
- Dr. Asha Shinde
Bombay Leprosy Project
Vidnyan Bhavan
Sion Chunabhatti
Mumbai 400 022, India
- Dr. Uday Khopkar
Department of Dermatology, Venereology and Leprology
BYL Nair Ch. Municipal Hospital
Mumbai Central, Mumbai 400 008, India
- Dr. V. V. Pai
Dr. R. Ganapati
Bombay Leprosy Project
Vidnyan Bhavan
Sion Chunabhatti
Mumbai 400 022, India
REFERENCES
1. Ebenezer, G. J., Suneeta, S. and Arunthathi, S. Clinical and histopathological activity in paucibacillary leprosy patients after fixed-duration multidrug therapy. Lepr. Rev. 68(1997)218-224.
2. Ganapati, R., Revankar, C. R., Pai, W. and Kingsley, S. Single dose treatment for paucibacil lary leprosy; feasibility of long-term follow up.(Letter). Int. J. Lepr. 67(1999)308-309.
3. Job, C. K., Jayakumar, J. and Aschhof, M. Delayed resolution versus treatment failure in paucibacillary leprosy patients under 6 months fixed drug therapy. Indian J. Lepr. 69(1997)131-142.
4. Multicentric Trial Group, India. Study of single dose chemotherapy in paucibacillary leprosy patients with two to three lesions.(Abstract)Int. J. Lepr. 66(1993)4A.
5. Pai, W., Bulchand, H. O., Revankar, C. R. and Ganapati, R. Single dose treatment for paucibacillary leprosy; clinical problems and management.(Letter)Int. J. Lepr. 67(1999)310-312.
6. Rajan, B. G., Edward, V. K., Gupte, M. D. et al. Efficacy of single-dose multidrug therapy for the treatment of single lesion paucibacillary leprosy. Lepr. Rev. 68(1997)341-349.
7. Revankar, C. R., Pai, V. V., Antony, M. S. and Ganapati, R. Single-dose treatment for paucibacillary leprosy; field implications(Letter). Int. J. Lepr. 67(1999)312-314.
8. Ridley, D. s. Numerical Indices in Skin Biopsy in Leprosy, Basle: CIBA-GEIGY Ltd. 1977, pp. 59-60.
9. Salunkhe, S. R., Pai, V. V. and Ganapati, R. Granuloma regression after fixed duration therapy. Indian J. Lepr. 70(1998)324.
10. Single Lesion Multicentre Trial Group. Efficacy of single dose multidrug therapy for the treatment of single lesion paucibacillary leprosy. Indian J. Lepr. 69(1997)121-130.