- Volume 68 , Number 4
- Page: 434–43
Antileprosy protective vaccination of sooty mangabey monkeys with BCG or BCG plus heat-killed Mycobacterium leprae: immunologic observations
ABSTRACT
Groups of sooty mangabey monkeys (SMM) were vaccinated and boosted with Mycobacterium bovis bacillus Calmette-Guerin (BCG), or BCG + low-dose (LD) or high-dose (HD) heat-killed M. leprae (HKML), or were unvaccinated. Prior to and following vaccination-boosting and subsequent M. leprae (ML) challenge, these and unvaccinated, unchallenged control monkeys were immunologically observed longitudinally for approximately 3 years. SMM [multibacillary (MB) leprosy-prone as a species] were not protected clinically by BCG or BCG + HKML, although the disease progress was slowed by vaccination with BCG alone. The longitudinal immune response profiles to BCG or BCG + HKML in SMM showed that: 1) vaccination with BCG or BCG + HKML initially stimulated significant in vitro blood mononuclear cell blastogenic responses to ML antigens, which returned to baseline post-boosting and post-live ML challenge; 2) BCG + LD HKML-vaccinated groups gave the largest blastogenic response (SI = 23) followed by the BCG + HD HKML group (SI = 14.5) and by the BCG-only vaccinated group (SI = 3.6); 3) significantly diminished numbers of blood CD4+ (helper) and CD4+CD29+ (helper-inducer) T-cell subsets were observed longitudinally in all ML-challenged groups compared to controls regardless of whether they had been vaccinated or not; 4) CD8+ (suppressor) T-cell numbers remained longitudinally constant, on average, in all ML-challenged groups (vaccinated or not) compared to controls; 5) there was a significant decrease in the CD4+:CD8+ ratio over time in all ML-challenged groups (vaccinated or not); 6) vaccination with BCG or BCG + LD or HD HKML resulted in significantly increased numbers of CD4+CD45RA+ (suppressor-inducer) T cells longitudinally compared to the unvaccinated, ML-challenged control group; and 7) over time, vaccination with BCG + HKML followed by live ML-challenge produced higher IGM:IgG antiphenolic glycolipid-I (PGL-I) serum antibody response ratios than BCG- only vaccinated, ML-challenged monkeys or unvaccinated, ML-challenged SMM, consistent with prior observations that IgG anti-PGL-I responses correlate with resistance to and protection from clinical leprosy and IgM anti-PGL-I responses correlate with increased susceptibility.RÉSUMÉ
groupes de singes Mangabey cendrés (SMM) furent primo-vaccinés et reçurent une injection de rappel de Mycobacterium bovis bacille de Calmette et Guérin (BCG), ou bien BCG + M. leprae inactivée à la chaleur (MLIC) à basse ou haute dose (BD et HD, respectivement), ou bien ne furent pas vaccinés. Avant, pendant et après la primo-vaccination, l'injection de rappel et l'épreuve virulente avec des M. leprae (ML), ces singes et des singes témoins non vaccînés et non-infectés furent suivis immunologiquement et longitudinalement pendant approximativement 3 années. Les SMM [très susceptibles à la lèpre multibacillaire (MB) comme caractéristique d'espèce] ne furent pas protégés cliniquement par le BCG et le BCG + MLIC, bien que l'évolution de la maladie fut retardée par la vaccination avec le BCG seul. Les profils longitudinaux de réponse immunitaire au BCG et au BCG + MLIC chez les SMM ont montré que : 1) la vaccination avec le BCG ou le BCG +MLIC a initialement provoqué une blastogenèse significative in vitro aux antigènes de ML, qui est ensuite retournée à un niveau basal après la vaccination de rappel et l'épreuve virulente par des ML vivantes; 2) le groupe vaccinés associant BCG + BD MLIC a montré la blastogenèse la plus importante (index de stimulation SI = 23) suivis par le groupe BCG + HD MLIC (SI = 14,5) puis par le groupe vacciné uniquement par le BCG (SI = 3,6); 3) une diminution significative du nombre des sous populations lymphocytaires T CD4+ (helper) et CD4+CD29+ (helper-inducteurs) fut observées longitudinalement chez tous les groupes inoculés par ML comparés aux témoins, qu'ils aient été vaccinés ou non; 4) Les nombres de lymphocytes T CD8+ restèrent constants au cours du temps, en moyenne, chez tous les groupes inoculés avec ML (vaccinés ou non) comparé aux témoins; 5) il y eut une diminution significative du ratio CD4+:CD8+ au cours du temps chez tous les groupes inoculés (vaccinés ou non); 6) la vaccination par le BCG ou BCG + BD MLIC a entraîné une augmentation significative du nombre de cellules T CD4+CD45RA+ (suppresseur-inducteur) longitudinalement comparé au groupe témoin non vacciné et inoculé par ML; et 7) au cours du temps, la vaccination avec le BCG + MLIC suivie par l'inoculation de ML vivantes a provoqué une réponse sérique en anticorps caractérisée par un ratio IgM : IgG anti-glycolipide phénolique de type I (PGL-I) plus élevée que les SMM vaccinés seulement par le BCG et inoculés par ML et les SMM non vaccinés et inoculés par ML, en accord avec les observations précédentes indiquant que les réponses à prédominance d'IgG anti-PGL-I sont corrélées avec résistance et protection contre la lèpre clinique tandis que les réponses à prédominance d'IgM anti-PGL-I sont corrélées à une susceptibilité accrue.RESUMEN
Varios grupos de monos mangabey pardos (MMP) se vacunaron y reestimularon con Mycobacterium bovis BCG, o con BCG mas una dósis baja (DB) o alta (DA) de M. leprae inactivado por calor (MLIC). o se mantuvieron sin vacunar. Todos los grupos de animales se estudiaron inmunológicamente antes y después de vacunarse, de reestimularse, y de infectarse con M. leprae viable. El seguimiento de los animales se hizo durante 3 años. Los MMP (altamente susceptibles de desarrollar lepra multibacilar, MB) no fueron protegidos clinicamente ni por BCG ni por BCG+MLIC, aunque el progreso de la enfermedad lue lento en los animales vacunados sólo con BCG. Los perfiles inmunológicos longitudinales en los grupos vacunados indicaron que: 1 ) la vacunación con BCG o BCG+MLIC inicialmente estimuló una significante proliferación in vitro de las células mononucleares de sangre periférica en respuesta a los antígenos de ML la cual luego regresó a los valores basales (post-reestímulo y post-infección eon ML); 2) el grupo vacunado con BCG+DB MLIC dio la mayor respuesta blastogénica (SI = 23), seguido por el grupo vacunado con BCG+DA MLIC (SI = 14.5) y luego por el grupo vacunado sólo con BCG (SI = 3.6); 3) en todos los grupos infectados con ML se observaron números significativamente reducidos de células sanguíneas T CD4+ (cooperadoras) y T CD4+CD29+ (inductoras), independientemente de si los animales fueron vacunados o no; 4) las células CD8+ (supresoras) permanecieron en números relativamente constantes en todos los grupos infectados con ML (vacunados o no), en comparación con los controles; (5) hubo una disminución significaiva en la relación CD4+:CD8+ en todos los animales infectados con ML (vacunados o no); 6) La vacunación con BCG o BCG+DB MLIC provocó un incremento significante en el número de células T CD4+CD45RA (supresoras-inductòras) en comparación con el grupo control infectado con ML pero no vacunado; y 7) la vacunación con BCG+MLIC seguida por la infección con ML vivo, indujo una mayor producción de anticuerpos IgG anti PGL-I que la vacunación sólo con BCG (e infección con ML) o que la infección con MLM sin vacunación previa. Esto es consistente con una observación anterior de que la respuesta IgG anti-PGL-I correlaciona con resistencia y protección contra la lepra clínica y que Ia respuesta IgM anti-PGL-I correlaciona con mayor susceptibilidad.We previously reported that rhesus monkeys (RM) (Macaca mulatta) are susceptible to paucibacillary (PB) forms of experimental leprosy in 75%-80% of leprosy-susceptible individuals (2, 8); whereas sooty mangabey monkeys (SMM) (Cercocebus atys) are prone to multibacillary (MB) forms in at least 80% of individuals (5, 7, 9). These differences provide the possibility of studying RM versus SMM comparatively as a means of learning more about the immunologic mechanisms involved in the predisposition of individuals toward the tuberculoid (TT) pole versus the lepromatous (LL) pole of leprosy. Moreover, using these models it is possible to examine the immunologic mechanisms of protective vaccination in individuals with predisposing susceptibility toward TT compared to those predisposed toward LL forms of leprosy. RM and SMM are phylo- genetically very similar to humans, and such studies should provide information pertinent to humans. We have carried out such a study using groups of Mycobacterium leprae (ML)-challenged RM and SMM which were unvaccinated or vaccinated with Bacillus Calmette Guerin (BCG) alone, or with BCG + low-dose (LD) heat-killed ML (HKML), or BCG + high dose (HD) I IKML. The clinical results of this study and the immunologic results from the RM groups have been previously reported (2, 3).
The clinical observations suggest that BCG offers some protection from clinical disease at both the paucibacillary (PB) and multibacillary (MB) ends of the leprosy spectrum; the combination of HKML with BCG, however, renders MB-prone SMM more susceptible to leprosy while enhancing the BCG protective effect in PB-prone RM (2).
Herein, we report some of the longitudinal immunologic observations spanning approximately 3 years in groups of SMM before any experimental manipulation, after vaccination with BCG, BCG + LD HKML or BCG + HD HKML, or no vaccination, after boosting with the same vaccines and after challenge with live ML.
MATERIALS AND METHODS
Animals. Details have been previously described (2, 3). Briefly, 35 sooty mangabey monkeys (SMM) (Cercocebus torquatus atys), aged 3-10 years, were purchased from the Yerkes Regional Primate Research Center's breeding colony in Atlanta, Georgia, U.S.A. (where they were born and reared). The SMM were divided into 4 ML-challenged experimental groups and one normal, unchallenged control group of 7 animals per group (2 females and 5 males/group). Three of the ML-challenged groups were vaccinated and boosted with BCG, BCG + LD HKML or BCG + HD HKML, respectively, while the fourth group was not vaccinated prior to ML-challenge.
Preparation of HKML for vaccination. Details were previously published (3). Briefly, SMM-origin ML was isolated from a SMM and inoculated into armadillos. Livers and spleens were taken from these armadillos when leprosy became sufficiently advanced and were stored frozen (-70°C) until dry-ice shipment to the laboratory of Dr. Patrick J. Brennan (Department of Microbiology, School of Veterinary Medicine, Colorado State University, Fort Collins, Colorado, U.S.A.) for isolation and purification of ML by the Draper method (1). The ML-preparations were heat-killed (auto-claved), lyophilized and shipped to the Tulane Regional Primate Research Center (TRPRC), Covington, Louisiana, U.S.A. These procedures were performed in Dr. Brennan's laboratory under contract AI- 52582 from the National Institutes of Allergy and Infectious Diseases.
Immunizations with BCG or BCG + HKML. Monkeys were vaccinated at the TRPRC with BCG alone or BCG + HD HKML or BCG + LD HKML by intracutaneous (i.c.) injection of 0.1 ml of the appropriate suspension (2). Primary vaccinations were followed by boosting at 7 weeks and challenge with live ML at 15 weeks. The three vaccine groups received the following vaccinations and boosters, respectively: 1) BCG-alone [1-2.6 × 106 viable units (VU)]; 2) BCG (1-2.6 × 106 VU) + LD HKML (1.6 × 109 ML); and 3) BCG (1-2.6 × 106 VU) + HD HKML (3.2 × 109 ML) (2). BCG viability, determined by Dr. Thomas M. Shinnick, Centers for Disease Control and Prevention (CDC), Atlanta, Georgia, U.S.A., was found to be at least 30%; more precise information was precluded by clumping.
Monkey inoculation. Monkeys were inoculated with live, freshly prepared ML suspensions by combined i.c. and intravenous (i.v.) routes using two i.c. sites per ear, the tip of the nose, outer forearms and outer calves; i.v. inoculations were made via the saphenous vein. ML for challenge had been obtained initially from a SMM (A015) with natural leprosy, and was successively sub-passaged through SMMs A022 and D173 prior to inoculation into armadillo #1742 at the Armed Forces Institute of Pathology, Washington, D.C., U.S.A., as previously described (2, 3). When leprosy was sufficiently advanced, the leproma, liver and spleen were removed from the armadillo and were shipped on wet ice overnight to the TRPRC. ML were re-isolated from the armadillo, as previously reported (2), and were used to challenge the vaccinated/boosted SMM. Inoculated ML had a morphologic index (MI) of 10% by the method of Shepard and McRae (13). Animals were inoculated i.c. using a total of 1.13 × 109 ML per animal equally distributed into the nine sites. Each SMM also received a total of 1.65 × 109 ML by the i.v. route. Thus, each SMM was challenged with a total of 2.78 × 109 ML by combined i.c. and i.v. routes.
ELISA. The assays were performed as previously reported (3, 4, 5, 9). Natural ML phenolic glycolipid-I (PGL-I), used as antigen (Ag), was provided by Dr. Patrick J. Brennan under NIH contract AI-52582. Data are presented as the group means ± 1 standard error of the means (S.E.M.) of longitudinal time points.
Blastogenesis. This procedure has been described in detail previously (3, 4, 8). Briefly, heparinized blood was used to prepare buffy coats which were centrifuged on Fi- coIl/Hypaque, washed and suspended in RPMI-1640 containing 20% heat-inactivated human AB serum, glutamine and penicillin/streptomycin. The peripheral blood mononuclear cell (PBMC) fraction was used at 2 × 106/ml for in vitro blastogenesis studies with or without 100 µg/ml of lepromin in U-bottom, 96-well, microtiter plates. Two x 105 PBMC per well were incubated at 37°C in 5% CO2, in triplicate for 5 days with stimulant or media prior to pulsing for 18 hr with 1 µci of 3H- thymidine per well. Thereafter, cells were washed and harvested on a cell harvester and quantified by scintillation counting. Stimulants were lepromin (human/armadillo, prepared by Dr. Wayne M. Meyers, Armed Forces Institute of Pathology, Washington, D.C., U.S.A.), Rees soluble ML antigen (gift from Dr. R. J. W. Rees, Medical Research Council, Middlesex, England) and ML r10Kd protein (provided by Dr. Patrick J. Brennan under NIH contract #AI-52582). The results are presented as group means of stimulation indices (SI) calculated, after subtraction of control values, by dividing the disintegrations per minute (dpm) in averaged triplicates in experimental tubes by the average triplicate dpm in the control (unstimulated) tubes. Data are presented as group means of longitudinal points (bars representing the S.E.M. are added to points of statistical importance; S.E.M. bars were not included for other points to make the graphs easier to read).
Peripheral blood lymphocyte (PBL) subsets. Whole EDTA blood was obtained longitudinally, stained with mouse antihuman monoclonal antibodies, and examined by flow cytometry, as previously reported (3, 4, 11, 12). Monoclonal antibodies with the following specificities were used: CD4+, CD8+, CD4+CD29+, CD4+CD45RA+ and CD8+. Results are presented as the absolute numbers of cells at different time points. S.E.M. bars were not added to these figures due to the problem of congestion, but the statistical data are noted where pertinent in the text.
Statistical analyses. All statistical calculations were performed using statistical programs for the Macintosh computer. Longitudinal comparisons between groups were performed by Mancova analysis. The paired t test was additionally used at selected time points for T-cell subset data analysis and for analysis of blastogenic data to compare group averages.
RESULTS
Longitudinal in vitro blastogenic responses are shown in Figure 1. These observations span a time period of approximately 3 years. The first two sets of points are baseline determinations prior to vaccination. The third set of points are 2 weeks post-vaccination; the fourth set are 2 weeks post-boosting and the fifth set represent 2 weeks post-inoculation (PI) with live ML. Large significant (p <0.0001) initial responses to vaccination were observed in the BCG + HKML groups (BCG + HD HKML, SI = 14.5; BCG + LD HKML, SI = 23), and a smaller significant response to BCG (SI = 3.6) occurred. These responses diminished rapidly after boosting and remained not significantly different from baseline values post-live ML-challenge, with the exception that the BCG + LD HKML group gave a significant post-challenge response (SI = 7.5). Similar patterns were seen in response to Rees' soluble ML antigen [2-week postvaccination (peak) responses: SI = 20, BCG + LD HKML; SI = 9, BCG + HD HKML]. ML 10-kDa protein also gave positive lower magnitude responses (data not shown), but no obvious pattern among ML-challenged groups could be discerned. During the 32-month PI period covered by these longitudinal studies, as previously detailed, the following numbers of SMM out of 7 per group showed clinical leprosy: unvaccinated, 7; BCG-only vaccinated, 6; BCG + LD HKML vaccinated, 7; and BCG + HD HKML vaccinated, 7 (2). The rate of progress of the disease was slowed in the BCG-only-vaccinated SMM group compared to the other three groups (2).
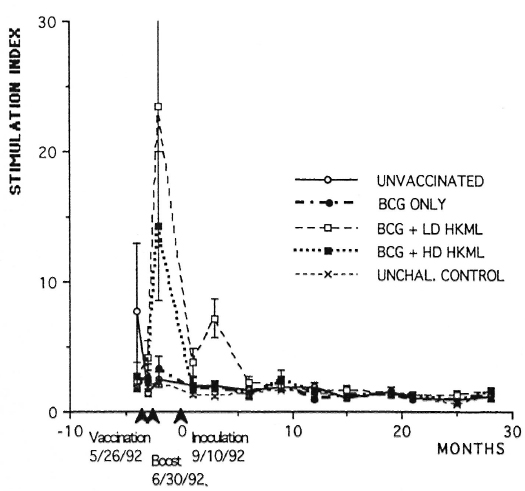
Fig. 1. Longitudinal in vitro blastogenic responses (stimulation indices, SI) of PBMC to lepromin in normal unchallenged (unchal) or ML-challenged unvaccinated or ML-challenged vaccinated groups of SMM.
Longitudinal monitoring of blood.T-cell subsets revealed significantly diminished numbers of CD4+ (helper) and CD4+ CD29+ (helper-inducer) subsets in all ML- challenged groups (vaccinated or unvacci- nated) during the 5-15-month PI period compared to the normal, unvaccinated, unchallenged group (Figs. 2 and 3). CD8+ (suppressor) cell numbers remained, on the average, constant over the period of study, with no significant differences between the ML-challenged groups (vaccinated versus control) and no difference between the unvaccinated, ML-challenged group and the unvaccinated, unchallenged normal control group (Fig. 4). The dynamics are reflected by there being a significant decrease in the CD4+:CD8+ ratio over time after M. leprae-challenge in all four ML-challenged groups (whether vaccinated or not) compared to normal controls over approximately 20 months PI (Fig. 5). The mean CD4+:CD8+ ratio in normal SMM is approximately 0.65 (Fig. 5).
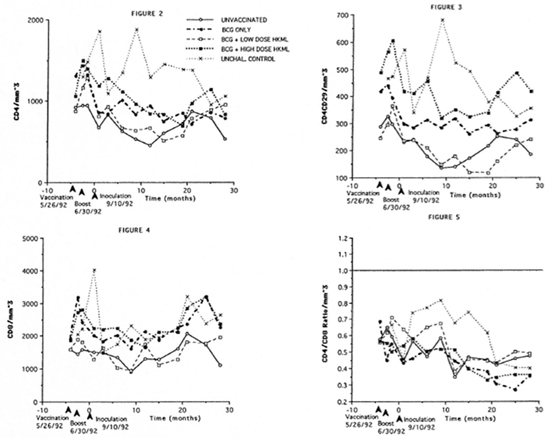
Fig . 2. Longitudinal peripheral blood lymphocyte CD4+ (helper) T-cell numbers/mm3 of blood by flow cytometry in the groups of SMM described in Figure 1. Fig. 3. Longitudinal peripheral blood lymphocyte CD4+CD29+ (helper-inducer) T-cell numbers/mm3 of blood by flow cytometry in the groups of SMM. Fig. 4. Longitudinal peripheral blood lymphocyte CD8+ (suppressor) T-cell numbers/mm3 of blood by flow cytometry (see Figs. 2 and 3). Fig. 5. Longitudinal peripheral blood lymphocyte CD4+:CD8+ ratios by flow cytometry (see Figs. 2-4).
Numbers of the CD4+CD45RA+ (suppressor-inducer) T-cell subset did not differ in vaccinated or unvaccinated, ML-challenged groups compared to the normal unvaccinated, unchallenged control group during this time frame (Fig. 6). However, when the unvaccinated, ML-challenged control group was compared to the vaccinated, ML-challenged groups (Mancova), significant increases (p <0.004) were observed in CD4+CD45RA+ numbers in the vaccinated groups for at least 1 year PI (Fig. 6).
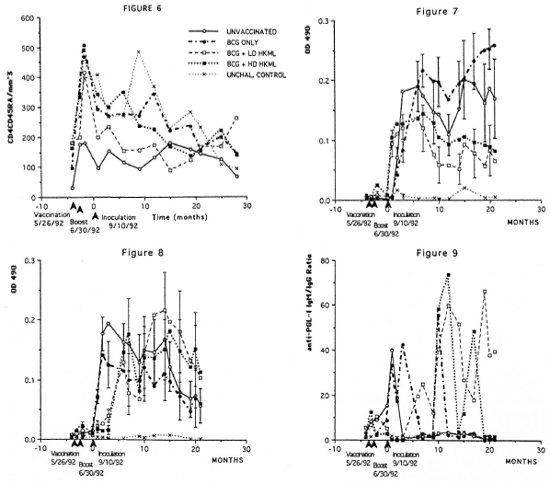
Fig. 6. Longitudinal peripheral blood lymphocyte CD4+CD45RA+ (suppressor-inducer) T-cell numbers/mm3 of blood by flow cytometry (see Figs. 2-4). Fig. 7. Longitudinal IgG anti-PGL-I serum antibody levels by ELISA in the groups of SMM. Fig. 8. Longitudinal IgM anti-PGL-I serum antibody levels (see Fig. 7). Fig. 9. Longitudinal ELISA-determined IgM:IgG serum anti-PGL-I antibody ratios.
Longitudinal ELISA determinations of IgG and IgM antibody to ML-specific PGL-I antigen showed the following (Figs. 7 and 8): a) no IgG or IgM anti-PGL-I response after vaccination or boosting over a 3.5-month period prior to ML inoculation; b) rapid responses of anti-PGL-I IgG and IgM after ML inoculation; c) within 6 months PI, the IgG anti-PGL-I levels began a sustained statistically significant (p <0.0006) drop in the BCG + LD HKML and the BCG + HD HKML groups, but remained elevated in the BCG-only-vaccinated group compared to the unvaccinated, ML-challenged group; and d) within 2 months PI, the IgM anti-PGL-I levels began a sustained drop in the BCG-only-vaccinated group compared to an overall significant increase (p <0.05) in the BCG + LD HKML and the BCG + HD HKML groups. After approximately 10 months PI, the BCG + LD HKML and the BCG + HD HKML groups showed sustained lgM:IgG ratios considerably in excess of 1, while the BCG- only-vaccinated and the unvaccinated, ML-challenged control groups maintained ratios of near 1 to less than 1 (Fig. 9).
DISCUSSION
Our prior observations showed that vaccination of SMM with BCG + LD or HD HKML failed to protect but, rather, exacerbated the disease; vaccination of SMM with BCG alone slowed the rate of progress of disease, but failed to significantly lower the eventual appearance of leprosy (2). Vaccination of RM with BCG or BCG + LD or HD HKML resulted in clear-cut protection from clinical leprosy (3). These differences between RM and SMM appear to be due to the fact that RM as a species are prone to the PB forms of leprosy in at least 75% of susceptible individuals (3, 8); whereas SMM are prone to MB leprosy in at least 80% of all individuals studied (2, 5, 7, 9, 10). These striking differences between RM and SMM suggested that comparative immunologic studies of vaccinated RM versus SMM might shed light on the mechanisms of an- tileprosy immunity not yet elucidated. Therefore, we followed longitudinally the immunologic responses of the RM and SMM from which the previous clinical data were generated (2). The immunologic results from the RM were previously reported (3). In the present study, immunologic results from the vaccinated SMM are presented and compared to the previously reported RM results.
In vitro blastogenic responses of SMM PBMC to lepromin were similar to those observed for the vaccinated RM (3). There was a strong response to lepromin 2 weeks post-vaccination in the SMM groups vaccinated with BCG + LD or HD HKML which returned toward baseline rapidly post- boosting and post-live ML-challenge. One striking difference observed between the species was that BCG + HD HKML-vacci- nated RM gave significantly greater blastogenic responses (SI = 28) than the group given BCG + LD HKML (SI = 13); whereas the opposite was true in SMM. In SMM, BCG + LD HKML gave the highest response (SI = 23) compared to BCG + HD HKML (SI = 14.5). In SMM, an additional smaller significant blastogenic response to lepromin was seen in the BCG + LD HKML group (SI = 7.5) 3 months post-live ML inoculation that was not significant in RM (3). In view of the fact that RM are protected from leprosy, whereas SMM are made more susceptible than normal SMM by vaccination with BCG + HKML (4), these dose-response differences between the two species suggest that SMM are more sensitive to sensitization by ML antigens and that the higher vaccine dose of HKML resulted in suppressed blastogenic responses by SMM PBMC.
Initial vaccination of SMM with BCG + HKML induced a rapid sensitization to ML antigens. Boosting approximately 4 weeks after vaccination failed to induce an anamnestic response; rather, responses rapidly diminished to baseline by approximately 14 weeks post-vaccination. We previously reported similar results in similarly vaccinated RM (3). However, vaccinated, ML-challenged RM continued to show small, intermittent, significant positive blastogenic responses to ML antigens compared to unvaccinated, ML-challenged RM over a 2-year period PI; whereas responses of vaccinated, ML-challenged SMM did not differ from the baseline responses of unvaccinated, ML-challenged or normal SMM during this period. The characteristics of the postbooster PBMC blastogenic response patterns suggest the possibility of antigen-induced suppression of blastogenic responsiveness in both species after the initial response to vaccination. Lepromatous leprosy-prone SMM appear to be highly responsive to ML antigens, which at the doses of HKML vaccines used here appeared to induce a potent systemic response by suppressor mechanisms to ML antigens which rendered SMM more susceptible to the progression of disease.
Longitudinal peripheral blood T-cell subset data revealed that, regardless of whether the animals were vaccinated or not, there was a significant decrease in the CD4+ (helper) and CD4+CD29+ (helper-inducer) numbers in all ML-challenged SMM compared to normal SMM; whereas the CD8+ subset remained constant. This scenario resulted in a decrease in the ratio of CD4+:CD8+ T cells over time. This ratio is naturally low in normal captive SMM from the Yerkes colony (0.65) compared to RM or humans (>1.0 in both species). Moreover, in ML-challenged RM, this initial ratio increases over time (4), especially in the BCG + HKML-vaccinated groups (3). Thus, RM as a species are able to maintain CD4+ T-cell numbers after inoculation with live ML, and this effect is enhanced by vaccination with BCG + HKML (3). Based on these observations, one apparent reason for leprosy susceptibility in SMM and for the failure of BCG or BCG + HKML vaccine to protect SMM is the failure to maintain CD4+ (and CD4+CD29+) subset numbers.
It is noteworthy that the numbers of the CD4+CD45RA+ (suppressor-inducer) subset increased in SMM after vaccination with BCG or BCG + LD or HD HKML and challenge with live ML, compared to the unvaccinated, ML-challenged group. This increase in CD4+CD45RA+ T-cell numbers was also seen in RM vaccinated with BCG + LD HKML, but not with BCG + HD HKML or BCG alone (3). In RM, this increase in CD4+CD45RA+ numbers as well as an increase in CD8+ (suppressor) cell numbers was offset by a concurrent increase in CD4+ and CD4+CD29+ subsets in the same groups (3); whereas similar significant increases in CD4+, CD4+CD29+ and CD8+ subsets were not seen in BCG or BCG + HKML-vaccinated, ML-challenged SMM. It is, therefore, possible that suppressor cell or suppressor-inducer T-cell effects are partly responsible for the observed increased leprosy susceptibility in SMM after vaccination with BCG + HKML compared to RM vaccinated similarly (3).
As observed in the RM vaccine studies (3) and other studies of leprosy in RM (8), SMM (5, 9) and chimpanzees (6), there is a consistent association in the present study between protection/susceptibility from/to leprosy after vaccination and the IgG versus IgM serum antibody responses to the ML-specific PGL-I antigen. Over the course of study, the IgM:IgG ratio of anti-PGL-I antibody evolved to much greater than 1 in SMM vaccinated with BCG + LD and HD HKML (which showed increased progression of lepromatous leprosy) compared to the group vaccinated with BCG alone (which showed slower progress of clinical leprosy) and compared to the unvaccinated group. In the RM vaccine study, groups vaccinated with BCG + HKML (which were highly protected) had the lowest IgM:IgG ratios followed by the BCG-alone-vaccinated group having the next highest and the unvaccinated, ML-challenged group which had the highest IgM:IgG anti-PGL-I ratio (4). Thus, the ratio of IgM:IgG anti-PGL-I serum antibody paralled the degree of susceptibility to leprosy in both RM and SMM after vaccination with BCG or BCG + HKML: the higher the susceptibility, the higher the IgM:IgG ratio. These responses are specific to PGL-I and are not reflective of nonspecific responses to mycobacterial antigens because parallel studies of responses to LAM gave independent results, as we have previously observed (9).
An interesting difference between RM and SMM was that RM vaccinated with BCG + LD or HD HKML began to produce anti-PGL-I antibodies after vaccination with BCG + HKML (3); whereas SMM failed to produce anti-PGL-I antibody responses after vaccination or after boosting with BCG + LD or HD HKML, but began production immediately after live ML-challenge in all vaccinated groups and the unvaccinated group. Thus, in terms of the humoral compartment of the immune system, RM express recognition of ML antigens when presented in the form of HKML (together with BCG); whereas SMM fail to express recognition of HKML antigens.
We do not yet know the significance of the correlation between the isotype of antibody response to PGL-I antigen and the clinical susceptibility to leprosy, but it is consistent in our hands in multiple studies among rhesus monkeys, sooty mangabey monkeys and chimpanzees (3, 5, 6, 8, 9). It is possible that this correlation is indicative of a direct involvement of the anti-PGL-I antibody in important antileprosy protective mechanisms or that it is merely an indirect reflection based on secondary responses to cytokine profiles resulting from other important pathways that are directly involved in protection from leprosy.
Acknowledgment. These studies were supported by a grant from the National Institutes for Allergy and Infectious Diseases (#AI-19302) and by a grant from the National Center for Research Resources (#RR-00164). We are indebted to the following persons for skillful technical assistance and data handling: Cynthia Trygg, Carol Coyne, Valerie Smith, Calvin Lanclos, Eva Pecunia and Doris O'Leary.
REFERENCES
1. Draper, P. Protocol 1/79: purification of M. leprae. Annex 1. Report of the Enlarged Steering Committee meeting, Geneva, 7-8 February 1979. Geneva: World Health Organization, 1979.
2. Gormus, B. J., Baskin, G. B., Xu, K., Bohm, R. P., Mack, P. A., Ratterree, M. S., Cho, S.-N., Meyers, W. M. and Walsh, G. P. Protective immunization of monkeys with BCG or BCG plus heat-killed Mycobacterium leprae: clinical results. Lepr. Rev. 69 (1998) 6-23.
3. Gormus, B. J., Baskin, G. B., Xu, K., Ratterree, M. S., Martin, L. N., Mack, P. A., Bohm, R. P., Jr., Meyers, W. M. and Walsh, G. P. Antileprosy protective vaccination of rhesus monkeys with BCG or BCG plus heat-killed Mycobacterium leprae: immunologic observations. Int. J. Lepr. 68 (2000) 27-39.
4. Gormus, B. J., Murphey-Corb, M., Martin, L. N., Baskin, G. B., Mack. P. A., Xu, K., Ratterree, M. S., Gerone, P. J., Scollard, D. M. and Gillis, T. P. Impaired responses to Mycobacterium leprae antigens in rhesus monkeys experimentally inoculated with simian immunodeficiency virus and M. leprae. Lepr. Rev. 69 (1998) 24-39.
5. Gormus, B. J., Ohashi, D. K., Ohkawa, S., Walsh, G. P., Meyers, W. M., Brennan, P. J. and Trygg, C. Serologic responses to Mycobacterium leprae-specific phenolic glycolipid-I antigen in sooty mangabey monkeys with experimental leprosy. Int. I Lepr. 56 (1988) 537-545.
6. Gormus, B. J., Xu, K. Y., Alford, P. L., Lee, D. R., Hubbard, G. B., Eichberg, J. W. and Meyers, W. M. A serologic study of naturally acquired leprosy in chimpanzees. Int. J. Lepr. 59 (1991) 450-457.
7. Gormus, B. J., Xu, K., Baskin, G. B., Martin, L. N., Bohm, R. P., Blanchard, J. L., Mack, P. A., Ratterree, M. S., McClure, H. M., Meyers, W. M. and Walsh, G. P. Experimental leprosy in monkeys. I. Sooty mangabey monkeys: transmission, susceptibility, clinical and pathological findings. Lepr. Rev. 66 (1995) 96-104.
8. Gormus, B. J., Xu, K., Baskin, G. B., Martin, L. N., Bohm, R. P., Jr., Blanchard, J. L., Mack, P. A., Ratterree, M. S., Meyers, W. M. and Walsh, G. P. Experimental leprosy in rhesus monkeys: transmission, susceptibility, clinical and immunological findings. Lepr. Rev. 69 (1998) 235-245.
9. Gormus, B. J., Xu, K., Cho, S.-N., Baskin, G. B., Bohm, R. P., Martin, L. N., Blanchard, J. L., Mach, P. A., Ratterree, M. S., Meyers, W. M. and Walsh, G. P. Experimental leprosy in monkeys. II. Longitudinal serological observations in mangabey monkeys. Lepr. Rev. 66 (1995) 105-125.
10. Gormus, B. J., Xu. K., Meyers, W. M., Walsh, G. P., Levis, W. R. and Meeker, H. C. Antibodies to lipoarabinomannan antigen in sooty mangabey monkeys experimentally inoculated with Mycobacterium leprae. Int. J. Lepr. 58 (1990) 65-72.
11. Martin, L. N., Murphey-Corb, M., Soike, F. K., Davison-Fairburn, B. and Baskin, G. B. Effects of initiation of 3'-azido, 3'-deoxythymidine (Zidovudine) treatment at different times after infection of rhesus monkeys with simian immunodeficiency virus. J. Infect. Dis. 168 (1993) 825-835.
12. Martin, L. N., Soike, K. F., Murphey-Corb, M., Bohm. R. P., Roberts, E. D., Kakuk, T. J., Thaisrivongs, S., Vidmar, T. J., Ruwart, M. J., Davio, S. R. and Tarpley, W. G. Effects of U-75875, a peptidomimetic inhibitor of retroviral proteases, on simian immunodeficiency virus infection in rhesus monkeys. Antimicrob. Agents Chemother. 38 (1994) 1277-1283.
13. Shepard, C. C. and McRae, D. H. A method for counting acid-fast bacteria. Int. J. Lepr. 36 (1968) 78-82.
1. Ph.D.; Department of Microbiology;
2. M.D.; Department of Microbiology;
3. Ph.D., Department of Microbiology;
4. D.V.M., Department of Pathology;
5. D.V.M.; Department of Veterinary Sciences, Tulane Regional Primate Research Center, 18703 Three Rivers Road, Covington, Louisiana 70433, U.S.A.
6. D.V.M.; Department of Veterinary Sciences, Tulane Regional Primate Research Center, 18703 Three Rivers Road, Covington, Louisiana 70433, U.S.A.
7. D.V.M., Department of Veterinary Sciences, Tulane Regional Primate Research Center, 18703 Three Rivers Road, Covington, Louisiana 70433, U.S.A.
8. M.D., Ph.D., Armed Forces Institute of Pathology,Washington, D.C., U.S.A.
9. Ph.D., American Leprosy Foundation, Rockville, Maryland, U.S.A.
Reprints request to Dr. Gormus at the above address or FAX 1-504-893-1352; e-mail: gormus@tpc.tulane.edu
Received for publication on 21 July 2000.
Accepted for publication on 20 September 2000.