- Volume 68 , Number 4
- Page: 444–51
Regulation of nitric oxide induced by mycobacterial lipoarabinomannan in murine macrophages: effects of interferon-β and Taurine-chloramine
ABSTRACT
We examined the effects of interferon beta (IFN-β) on the production of liporabinomannan (LAM)-induced nitric oxide (NO) in peritoneal macrophages from low- responder and high-responder (C3H/HeJ and C3H/OuJ) mice. NO was produced in a dose response when induced by lipo- polysaccharide (LPS) or LAM plus interferon gamma (IFN-γ) or IFN-β in both high- and low-responder mice. In contrast to IFN-γ, both high- and low-responder mice failed to induce nitrite production when IFN-β was added, except at a high concentration of IFN-β. Tau-Cl (0.5 mM) inhibited NO production about 50% in the high-responder strain when cells were activated with LPS or LAM in combination with either IFN-β or IFN-γ, and almost abolished NO production at 1.0 mM. In the low-responder strain, Tau-Cl (0.5 mM) significantly inhibited NO production when cells were activated with IFN-γ or IFN-β in addition to LPS or LAM, but did not completely inhibit NO production at 1.0 mM. Tau-Cl appears to play a potent role in regulating inflammatory reaction-induced bacterial or mycobacterial organisms. These data indicate a pivotal role for IFN-γ and IFN-β for the production of LPS and LAM initiated NO in peritoneal macrophages from low-responder (C3H/HeJ) mice.RÉSUMÉ
Nous avons examiné l'effet de l'interféron bêta (IFN-β) sur la production de monoxyde d'azote (NO) induite par ie lipoarabinomannane (LAM) de macrophages péritonéaux de souris de souches faiblement et hautement productrices (souches C3H/HeJ et C3H/OuJ respectivement). NO fut produit de manière dose dépendante lorsque les macrophages furent stimulés par du lipopolysaccharide (LPS), ou bien du LAM associé à de l'IFN-γ ou de IFN-β, á la fois chez les souris hautement et faiblement productrices. Cependant, contrastant avec l'IFN-γ, tant les souris hautement que faiblement productrices ont été incapables d'induire la production de NO lorsque l'IFN-β seule à été ajouté dans le milieu de culture, sauf à forte concentration. Tau-Cl (0,5 mM) a inhibé la production de NO d'environ 50% chez la souche hautement productrice lorsque les cellules furent stimulées par le LPS ou le LAM combiné à l'IFN-γ ou á l'IFN-β, et l'a presque entièrement annulé à 1,0 mM. Parmi les souris faiblement productrices, Tau-Cl (0,5 mM) a inhibé de façon statistiquement significative la production de NO lorsque les cellules furent activées soit par l'IFN-γ, soit par l'IFN-β ajouté au LPS ou au LAM, mais ne l'a pas complètement inhibé à 1,0 mM. Tau-Cl semble jouer un rôle important dans l'homéostasie de la réaction inflammatoire induite par les bactéries et les mycobactéries. Ces données montrent les rôles très importants de IFN-γ et de l'IFN-β pour la production de NO induite par LPS et LAM, par les macrophages péritonéaux de souris faiblement productrices (C3H/HeJ).RESUMEN
En este estudio investigamos el efecto del interferón beta (IFNβ-) sobre la producción de óxido nítrico (NO) inducido con lipoarabinomanana (LAM) en los macrófagos peritoneales de ratones respondedores bajos (C3H/HeJ) y altos (C3H/OuJ). El NO se produjo con un patrón de dosis-respuesta cuando se indujo con lipopolisacárido (LPS) o LAM más interferón gamma (IFN-γ) o con IFN-β, tanto en los ratones respondedores altos como en los bajos. En contraste con lo ocurrido con el IFN-γ, ni los ratones respondedores altos ni los bajos fueron capaces de producir nitrito cuando se adicionó IFN-β, excepto a una alta concentración del mismo. La adición de Tau-Cl (0.5 mM) inhibió la producción de NO casi al nivel del 50% en la cepa respondedora alta cuando las células se activaron con LPS o LAM en combination con IFN-β o IFN-γ, y casi eliminó la producción de NO a la concentration 1 mM. En la cepa respondedora baja, Tau-Cl (0.5 mM) inhibió significativamente la producción de NO cuando las células fueron activadas con IFN-γ o IFN-β además de LPS o LAM pero no inhibió por completo la producción de NO a la concentración 1.0 mM. Tau-Cl parece jugar un papel importante en la regulación de la respuesta inllamatoria inducida por microorganismos bacterianos o micobacterianos. Estos datos indican un papel primordial del IFN-γ y el IFN-β en la producción de NO inducida con LPS y LAM en los macrófagos peritoneales de los ratones respondedores bajos (C3H/HeJ).Nitric oxide (NO) is a potent mediator of a variety of biological phenomena and has been implicated in the killing of a wide variety of intracellular organisms including mycobacteria (1, 2, 6, 11, 13). At high concentrations, NO, often in conjunction with tumor necrosis factor-alpha (TNF-α), has been implicated in autoimmune and other inflammatory tissue destruction, including the manifestations of septic shock (29, 30). Certainly, the regulation of the levels of NO allowing for physiologic function but avoiding pathologic destruction is among the central questions of immunology today. Bacterial lipopolysaccharide (LPS) is a major stimulant of mouse inducible nitric oxide synthase (iNOS) which has been shown to be augmented by a variety of cytokines (29, 30, 33) and tightly downregulated by a second group of cytokines, such as transforming growth factor-beta (TGF-β), interleukin-10 (IL-10), and IL-4 (4, 15, 43). Mycobacteria possess their own LPS, namely lipoarabinomannans (LAM) that, like LPS, are able to activate murine iNOS (1, 7, 8, 13). Indeed, there is good evidence that NO is a major effector molecule for mycobacterial killing in the mouse and possibly humans (11, 12, 21, 31). Recently interferon beta (IFN-β) has been implicated as an additional functionally separate signal from gamma interferon (IFN-γ) in LPS-initiated NO production by mouse macrophages (14, 45).
Taurine protects tissues from damage caused by overt inflammatory responses in a variety of model systems (16, 17, 40). Although the mechanism(s) of taurine protection is not well understood, much attention has been focused on the ability of taurine to attenuate the indiscriminate cellular damage caused by HOC1/OCl- through formation of taurine chloramine (Tau-Cl) (5, 18, 20). The formation of Tau-Cl may also be catalyzed directly by the halide-dependent myeloperoxidase that is associated with polymorphonuclear leukocytes (PMN), eosinophils and basophils (44). Tau-Cl has been shown to inhibit production of NO, TNF-α and prostaglandin E2 (PGE2) by macrophages activated by LPS in culture (33, 37). Production of NO and TNF-α in a LAM-activated macrophage cell line was also suppressed by Tau-Cl (41). Tau-Cl exerts its influence on the production of these inflammatory mediators through mechanisms that involve transcriptional and post- transcriptional events (35). These findings have led to the suggestion that Tau-Cl may function as a biomodulator of the inflammatory response and may account for some of the tissue protective attributes associated with taurine.
Macrophage activation by LPS or LAM requires binding of LPS or LAM with high affinity to CD14, a glycosyl phosphatidyl- inositol-linked protein expressed on the surface of macrophages, and to toll-like receptor 2 and/or 4 protein (TLR 2 and/or TLR4) (22, 25). Substrains of the C3H mouse diverge in their responsiveness to LPS. C3H/OuJ, lpsn (high-responder strain) gives a vigorous response to LPS, while the C3H/HeJ, lpsd (low-responder strain) has a single mutation in tlr4 and is hyporesponsive to LPS (23, 25, 36).
In the present study, we examine the role of IFN-β for both LPS- and LAM-induced NO production in LPS high-responder and LPS low-responder strains of mice. NO production in both strains is downregulated by Tau-Cl.
MATERIALS AND METHODS
Animals. C3H/OuJ (LPS high responder, lpsn) and C3H/HeJ (LPS low responder, lpsd) female mice (8-12 weeks old) were purchased from Jackson Laboratories (Bar Harbor, Maine, U.S.A.). Animals were kept with free access to food and water.
Reagents. Ara-LAM (Mycobacterium sp. rapid growing or Mycobacterium tuberculosis H37Ra) was kindly provided by Dr. John T. Belisle (Colorado State University, Fort Collins, Colorado, U.S.A.). Recombinant murine IFN-γ, RPMI 1640, Hanks balanced salt solution (HBSS) without Ca2+ and Mg2+, fetal calf serum (FCS), penicillin, streptomycin, and glutamine were purchased from Gibco BRL (Gaithersburg, Maryland, U.S.A.). IFN-β was obtained from Lee Biomolecular Research Laboratories, Inc., San Diego, California, U.S.A. The endotoxin content was measured by Limulus test kits (Biowhittaker, Inc., Wak- ersville, Maryland, U.S.A. and Associates of Cape Cod, Inc., Woods Hole, Massachusetts, U.S.A.). Dulbecco's minimal essential medium (DMEM), taurine, and sodium hypochlorite (NaOCl) were obtained from Sigma Chemical Company (St. Louis, Missouri, U.S.A.). Tau-Cl was synthesized and monitored in our laboratory as previously described (34).
Cell culture preparation. Murine peritoneal exudate cells were collected from the abdominal cavity with Ca++, Mg++ free HBSS 4 days after 1 ml of an intraperitoneal injection of 5% thioglycollate broth (Difco, Detroit, Michigan, U.S.A.). The NO assay was conducted using complete medium: DMEM, 10% FCS, 2 mM glutamine, penicillin and streptomycin without phenol red. Cells were washed with complete medium and 2 × 105 cells/well were used in 96-well plates (Corning Glass Works, Corning, New York, U.S.A.). After overnight adherence in 5% CO2, nonadherent cells were removed. Differential staining confirmed that the remaining cells were macrophages. Stimulators such as LPS, ara-LAM, rIFN-γ, and rIFN-β were added in a final volume of 200 µl. After 24 hr, 100 µl of the supernatant was used from each well for 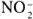 determination.
determination.
Nitrite measurement. Nitrite ( ) concentration was measured in 100 µl aliquots of conditioned medium from the 96-well plates by incubating with an equal volume of Greiss reagent (1% sulfanilamide + 0.1% naphtylethylene diamine di-hydrochloride + 2.5% phosphoric acid) at room temperature for 10 min in a microplate as previously described (33).
) concentration was measured in 100 µl aliquots of conditioned medium from the 96-well plates by incubating with an equal volume of Greiss reagent (1% sulfanilamide + 0.1% naphtylethylene diamine di-hydrochloride + 2.5% phosphoric acid) at room temperature for 10 min in a microplate as previously described (33).
Statistical analysis. The differences between groups were determined by Student's paired t test and analysis of variance techniques (Statistica; Statsoft, Inc., Tulsa, Oklahoma, U.S.A.). To control for multiple group comparisons, the Scheffe pairwise method was employed. Both statistical tests resulted in the same group differences at the level of p <0.05.
RESULTS
IFN-γ augmented the LPS (10 µg/ml)-induced production of nitrite in both strains of mice at all concentrations employed (Fig. 1 A). In the absence of IFN-γ, no nitrite production was detected in the low-responder strain (C3H/HeJ). At high doses of IFN-γ (10 and 50 U/ml), the low-responder (C3H/HeJ) strain approached the maximum nitrite production achieved by the high-re- sponder (C3H/OuJ) cells (p <0.001). IFN-β exhibited augmentation of the LPS-induced nitrite production in both strains only at high concentrations (1000 U/ml) (p <0.001) (Fig. 1B). While the high-responder strain (C3H/OuJ) achieved in excess of 14 nmoles nitrite/well, the low-responder (C3H/HeJ) cells were only in excess of 4 nmoles nitrite/well even at this very high dose of IFN-β.
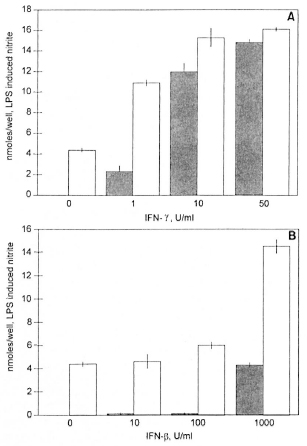
Fig. 1. Nitrite production induced by LPS (10 µg/m1) plus IFN-γ (A) and IFN-β (B) in peritoneal macrophages from high-responder mice (C3H/OuJ,  ) and low-responder mice (C3H/HeJ,
) and low-responder mice (C3H/HeJ,  ) Data is mean ± S.D. of triplicate samples. This experiment is representative of three independent experiments. Thioglycollate-elicited peritoneal macrophages were activated for 24 hr. NO was measured as nitrite using the Greiss reagent. Control levels without IFN-γ or IFN-β in C3H/HeJ were negligible.
) Data is mean ± S.D. of triplicate samples. This experiment is representative of three independent experiments. Thioglycollate-elicited peritoneal macrophages were activated for 24 hr. NO was measured as nitrite using the Greiss reagent. Control levels without IFN-γ or IFN-β in C3H/HeJ were negligible.
LAM failed to induce significant nitrite production in the nonresponder strain (C3H/HeJ) in the absence of IFN-γ; whereas the responder strain (C3H/OuJ) produced over 3 nmoles/well of nitrite without IFN-γ (Fig. 2A). However, with as little as 1 U/ml of IFN-γ, the nonresponder (He/J) macrophages produced in excess of 5 nmoles/well of nitrite and at 10 to 50 U/ml of IFN-γ produced 12 and 14 nmoles/well nitrite. These levels are comparable to the high-responder (C3H/OuJ) macrophages (Fig. 1A).
Fig. 2. Nitrite production induced by LAM (10 µg/m1) plus IFN-γ (A) and IFN-β (B) in peritoneal macrophages from high-responder mice (C3H/OuJ,  ) and low-responder mice (C3H/HeJ,
) and low-responder mice (C3H/HeJ,  ). Data is mean ± S.D. of triplicate samples. This experiment is representative of three independent experiments. Thioglycollate-elicited peritoneal macrophages were activated for 24 hr. NO was measured as nitrite using the Greiss reagent. Control levels without IFN-γ or IFN-β were negligible.
). Data is mean ± S.D. of triplicate samples. This experiment is representative of three independent experiments. Thioglycollate-elicited peritoneal macrophages were activated for 24 hr. NO was measured as nitrite using the Greiss reagent. Control levels without IFN-γ or IFN-β were negligible.
Similarly, the low-responder (C3H/HeJ) macrophages failed to respond to LAM alone but showed significant (p <0.001) levels of nitrite when 1000 U/ml of IFN-β was added (Fig. 2B). Low doses of IFN-β produced barely detectable amounts of LAM-induced nitrite in the nonresponder strain (He/J) and failed to augment the response in the responder (C3H/OuJ) strain. However, highly significant (p <0.001) levels of LAM-induced nitrite were produced in both the C3H/HeJ and C3H/OuJ macrophages at high concentrations (1000 U/ml) of IFN-β (10 and 11.8 nmoles/well, respectively) (Fig. 2B).
In high-responder mice, Tau-Cl was a potent downregulator of both LPS (10 µg/ml)- and LAM (10 µg/ml)-induced nitrite production whether augmented by IFN-γ (50 U/ml) or by IFN-β (1000 U/ml) (Fig. 3A and B). Tau-Cl at 0.5 mM produced about 50% inhibition of NO production and almost complete inhibition at 1.0 mM (p <0.001). In low-responder mice, Tau-Cl significantly (p <0.001) inhibited nitrite production induced by LPS or LAM plus IFN-γ or IFN-β in a somewhat similar pattern (Fig. 4A and B). When peritoneal macrophages were activated with IFN-β in addition to LPS or LAM, Tau-Cl (0.5 mM) remarkably inhibited nitrite production (22.2% vs 10.1%, respectively). It was also notable that Tau-Cl at 1.0 mM did not abolish nitrite production in low-responder mice when cells were activated with IFN-γ in addition to LPS or LAM (20% vs 11.7%, respectively).
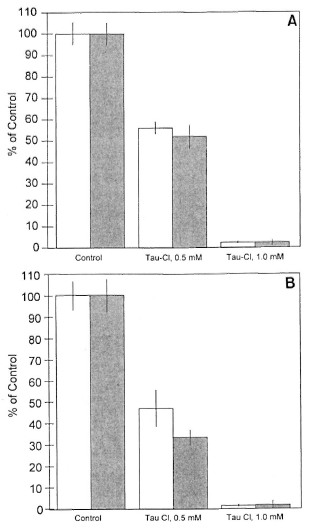
Fig. 3. Inhibition of nitrite production by Tau-Cl in peritoneal macrophages from high-responder macrophages. NO production was induced by LPS (A) or LAM (B) plus IFN-γ (  ) or IFN-β (
) or IFN-β (  ). Macrophages activated by LPS (10 µg/ml) plus IFN-γ (50 U/ml) or IFN-β (1000 U/ml) produced 14.4 ± 0.8 and 8.5 ± 0.4 nmoles/well, respectively. Macrophages activated by LAM (10 µg/ml) plus IFN-γ (50 U/ml) or IFN-β (1000 U/ml) produced 13.1 ± 0.9 and 5.7 ± 0.5 nmoles/well, respectively. The data are represented as % of control ± S.D. This experiment is representative of three independent experiments.
). Macrophages activated by LPS (10 µg/ml) plus IFN-γ (50 U/ml) or IFN-β (1000 U/ml) produced 14.4 ± 0.8 and 8.5 ± 0.4 nmoles/well, respectively. Macrophages activated by LAM (10 µg/ml) plus IFN-γ (50 U/ml) or IFN-β (1000 U/ml) produced 13.1 ± 0.9 and 5.7 ± 0.5 nmoles/well, respectively. The data are represented as % of control ± S.D. This experiment is representative of three independent experiments.
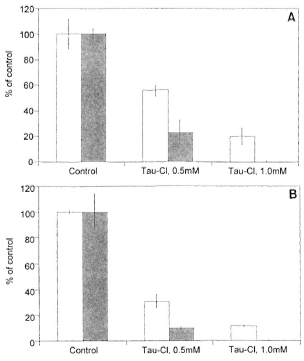
Fig. 4. Inhibition of nitrite production by Tau-Cl in peritoneal macrophages from low-responder macrophages. NO production was induced by LPS (A) or LAM (B) plus IFN-γ (  ) or IFN-β (
) or IFN-β (  ). Macrophages activated by LPS (10 µg/ml) plus IFN-γ (50 U/ml) or IFN-β (1000 U/ml) produced 7.5 ± 0.9 and 6.0 ± 0.3 nmoles/well, respectively. Macrophages activated by LAM (10 µg/ml) plus IFN-γ (50 U/ml) or IFN-β (1000 U/ml) produced 12.2 ± 0.2 and 4.3 ± 0.6 nmoles/well, respectively. The data are represented as % of control ± S.D. This experiment is representative of three independent experiments.
). Macrophages activated by LPS (10 µg/ml) plus IFN-γ (50 U/ml) or IFN-β (1000 U/ml) produced 7.5 ± 0.9 and 6.0 ± 0.3 nmoles/well, respectively. Macrophages activated by LAM (10 µg/ml) plus IFN-γ (50 U/ml) or IFN-β (1000 U/ml) produced 12.2 ± 0.2 and 4.3 ± 0.6 nmoles/well, respectively. The data are represented as % of control ± S.D. This experiment is representative of three independent experiments.
DISCUSSION
Our findings are in keeping with Zhang, et al. (45) and Fujihara, et al. (14) who found IFN-β to be an important cofactor for LPS-induced macrophage iNOS. Our current study extends the role of IFN-β to mycobacteria. We found IFN-β (1000 U/ml) synergized NO production with LPS as well as mycobacterial-derived LAM. IFN-β restored NO production in low-responder mice to a level similar to that in high-responder mice. Our study demonstrates that levels of NO induced by either mycobacterial LAM or LPS in combination with IFN-β are significantly inhibited by Tau-Cl. Due to daily biological variations of primary cultures, the production of NO was variable (4.0-16.0 nmole/well in Figs. 1, 2, 3 and 4) (19). Tau-Cl inhibited production of NO at all doses in this study. The range of Tau-Cl examined in this study was not toxic to murine peritoneal macrophages, which was confirmed using trypan blue exclusion (19). In our previous studies, Tau-Cl inhibited the in vitro production of proinflammatory mediators such as NO, TNF-α, PGE2, in activated murine macrophages or macrophage cell lines (33, 37) and IL-6 and IL-8 in activated human PMN (32). Interestingly, NOS was inhibited by Tau-Cl at the transcriptional level in contrast to TNF-α and COX-2 which were inhibited at the translational level (35, 37).
While there is little doubt that NO and reactive nitrogen intermediates (RNI) play a role in the killing of a variety of intracellular organisms in murine macrophages, the role in human macrophages is controversial. Indeed, it has been reported that a divergence in evolution has led to a lack of conservation of human macrophage iNOS (39). Adequate evidence exists for the presence of a high output NO synthase in human macrophages (11, 12, 21). Indeed, a cDNA for iNOS has been cloned from human macrophages (38). Clearly, differences between human and murine iNOS exist in terms of requirements for activation. Live microbes may be important along with different co-factors, different cytokines and various other endogenous modulators of gene transcription and enzyme activity.
Leprosy is the original TH1-TH2 paradigm (24, 26, 42). The ieprosy model of TH1 and TH2 has been extended to leishmaniasis (27) and a wide variety of other diseases (24). More recently it has been recognized that the initial encounter of microbial antigens by the innate immune system can affect or influence the subsequent induction of either the TH1 or TH2 limb of the acquired immune system (23). Indeed, at least nine homologs of the Drosophila toll protein, which are TLR-like receptors, have been identified in humans (9, 23, 28), and the toll-like receptor has been identified as a major receptor for LPS and LAM (25). Previous studies have shown that carbohydrate structure is related to mycobacterial pathogenesis with virulent strains showing a mannose capping (2, 3, 8). Future studies will address the possible relationship of toll receptors to carbohydrate structure. The C3H/HeJ mouse has a point mutation of the toll-like receptor 4 gene (tlr4) (25, 36). It is likely that a human homolog of the murine C3H/HeJ mutation exists. The finding that mycobacterial LAM shows the same signaling defect in the C3H/HeJ mouse that has been known for LPS, demonstrates the importance of future studies of mutations of the toll-like receptors in human mycobacterial diseases.
Acknowledgment. This work was supported by grants from the New York Lung Association, Staten Island University Hospital, and the Office of Mental Retardation and Developmental Disabilities of New York State.
REFERENCES
1. Adams, L. B., Franzblau, S. G., Vavrin, Y., Hibbs, J. B. and Krahenbuhl, J. L-arginine-dependent macrophage effector functions inhibit metabolic activity of Mycobacterium leprae. J. Immunol. 147 (1991) 1692-1696.
2. Adams, L. B., Fukutomi, Y. and Krahenbuhl, J. L. Regulation of murine macrophage effector functions by lipoarabinomannan from mycobacterial strains with different degrees of virulence. Infect. Immun. 61 (1993) 4173-4181.
3. Anthony, L. S., Chatterjee, D., Brennan, P. J. and Nano, F. E. Lipoarabinomannan from Mycobacterium tuberculosis modulates the generation of reactive nitrogen intermediates by gamma interferon-activated macrophages. FEMS Immunol. Med. Microbiol. 4 (1994) 299-305.
4. Bogdan, C., Vodovotz, Y, Paik, J., Xie, Q. W. and Nathan, C. Mechanism of suppression of nitric oxide synthase expression by interleukin-4 in primary mouse macrophages. J. Leuk. Biol. 55 (1994) 227-233.
5. Cantin. A. M. Taurine modulation of hypochlorous acid-induced lung epithelial cell injury in vitro. J. Clin. Invest. 93 (1994) 606-614.
6. Chan, J., Xing, Y., Magliozzo, R. S. and Bloom, B. R. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J. Exp. Med. 175 (1992) 1111-1122.
7. Chatterjee, D. and Khoo, K. H. Structural definition of the non-reducing termini of mannosecapped LAM from Mycobacterium tuberculosis through selective enzymatic degradation and fast atom bombardment-mass spectrometry. Glycobiology 3 (1993) 497-506.
8. Chatterjee, D., Lowell, L., Rivoire, B., McNeil, M. R. and Brennan, P. J. Lipoarabinomannan of Mycobacterium tuberculosis capping with mannosyl residues in some strains. J. Biol. Chem. 267 (1992) 6234-6239.
9. Chuang, T. H. and Ulevitch, R. J. Cloning and characterization of a sub-family of human toll-like receptors: hTLR7, hTLR8 and hTLR9. Eur. Cytokine Netw. 3 (2000) 372-378.
10. Denis, M. Interferon-gamma-treated murine macrophage inhibit growth of tubercle bacilli via the generation of reactive nitrogen intermediates. Cell. Immunol. 132 (1991) 150-157.
11. Denis, M. Human monocytes/macrophages: NO or no NO? J. Leuk. Biol. 55 (1994) 682-683.
12. Dumarey, C. H., Labrousse, V., Rastogi, N., Vargaftig, B. B. and Bachelet, M. Selective Mycobacterium avium-induced production of nitric oxide by human monocyte-derived macrophages. J. Leuk. Biol. 56 (1994) 36-40.
13. Flesch, S. A. and Kaufmann, S. Mechanisms involved in mycobacterial growth inhibition by gamma interferon-activated bone marrow macrophages: role of reactive nitrogen intermediates. Infect. Immun. 59 (1991) 3213-3218.
14. Fujihara, M., Ito, N., Pace, J., Watanake, Y., Russell, S. and Suzuki, T. Role of endogenous interferon-β in lipopolysaccharide-triggered activation of inducible nitric oxide synthase gene in a mouse macrophage cell line J774. J. Biol. Chem. 269 (1994) 12773-12778.
15. Gazzinelli, R. T., Oswald. I. P., James, S. L. and Sher, A. IL-10 inhibits parasite killing and nitrogen oxide production by rlFN-γ-activated macrophages. J. Immunol. 148 (1992) 1792-1796.
16. Gordon, R. E., Park, E., Laskin, D. and Schuller-Levis, G. B. Taurine protects rat bronchioles from acute ozone exposure: a freeze fracture and electron microscopic study. Exp. Lung Res. 24 (1998) 659-674.
17. Gould, R. E., Shaked, A. A. and Solano, D. F. Taurine protects hamster bronchiols from acute NO2-induced alteration. Am. J. Pathol. 125 (1986) 585-600.
18. Grisham, M. B., Jefferson, M. M., Melton, D. F. and Thomas, E. L. Chlorination of endogenous amines by isolated neutrophils. J. Biol. Chem. 269 (1984) 10404.
19. Kim, C., Park, E., Quinn, M. R., and Schuller-Levis, G. The production of superoxide anion and nitric oxide by cultured murine leukocytes and the accumulation of TNT-α in the conditioned media is inhibited by taurine chloramine. Immunophar- macology 34 (1996) 89-95.
20. Marques, L. A. and Dunford, H. B. Chlorination of taurine by myeloperoxidase: kinetic evidence for an enzyme-bound intermediate. J. Biol. Chem. 269 (1994) 7950-7956.
21. Mautino, G., Paul-Eugene, N., Chanez, P., Vignola, A. M., Kolb, J. P., Bousquet, J. and Dugas, B. Heterogeneous spontaneous and interleukin-4 induced nitric oxide production by human monocytes. J. Leuk. Biol. 56 (1994) 15-20.
22. Medvedev, A. E., Kopydlowski, K. M. and Vogel, S. N. Inhibition of lipopolysaccharide-induced signal transduction in endotoxin-tolerized mouse macrophages: dysregulation of cytokine, chemokine, and toll-like receptor 2 and 4 gene expression. J. Immunol. 11 (2000) 5565-5574.
23. Medzhitov, R., Preston-Hurlburt, P. and Janeway, C. A., Jr. A human homologue of the Drosophila toll protein signals activation of adaptive immunity. Nature 388 (1997) 394-397.
24. Modlin, R. L. Th1-TH2 paradigm: insights from leprosy. J. Invest. Dermatol. 102 (1994) 828-832.
25. Modlin, R. L., Brightbill, H. D. and Godowski, P. J. The toll of innate immunity on microbial pathogens. N. Engl. J. Med. 340 (1999) 1834-1835.
26. Morel, P. A. and Oriss, T. B. Cross coregulation between Th1 and Th2 cells. Crit. Rev. Immunol. 18 (1998) 273-303.
27. Mosmann, T. R., Cherwinski, H., Bond, M. W., Giedlin, M. A. and Coffman, R. L. Two types of murine helper T cell clones. 1. Definitions according to profiles of lymphokine activities and secreted proteins. J. Immunol. 136 (1986) 2348-2357.
28. Muzio, M., Polentarutti, N., Bosisio, D., Prahladan, M. K. and Mantovani, A. Toll-like receptors: a growing family of immune receptors that are differentially expressed and regulated by different leukocytes. J. Leukoc. Biol. 4 (2000) 450-456.
29. Nathan, C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 6 (1992) 3051-3064.
30. Nathan, C. and Hibbs, J. B., Jr. Role of nitric oxide synthesis in macrophage antimicrobial activity. Curr. Opin. Immunol. 3 (1991) 65-70.
31. Pagett, E. L. and Pruett, S. B. Evaluation of nitrite production by human monocyte-derived macrophages. Bochem. Biophys. Res. Commun. 186 (1992) 775-781.
32. Park, E., Alberti, J., Quinn, M. R. and Schuller-Levis, G. Taurine chloramine inhibits the production of superoxide anion, IL-6 and IL-8 in activated human polymorphonuclear leukocytes. Adv. Exp. Med. Biol. 442 (1998) 177-182.
33. Park, E., Quinn, M. R., Wright, C. E. and Schuller-Levis, G. Taurine chloramine inhibits the synthesis of nitric oxide and the release of tumor necrosis factor in activated RAW 264.7 cells. J. Leuk. Biol. 54 (1993) 119-124.
34. Park, E., Schuller-Levis, G., Jia, J. and Quinn, M. R. Preactivation exposure of RAW 264.7 cells to taurine chloramine attenuates subsequent production of nitric oxide and expression of iNOS mRNA. J. Leuk. Biol. 61 (1997) 161-166.
35. Park, E., Schuller-Levis, G. and Quinn, M. R. Taurine chloramine inhibits production of nitric oxide and TNF-α in activated RAW 264.7 cells by mechanisms that involve transcriptional and translational events. J. Immunol. 154 (1995) 4778-4784.
36. Poltorak, A., He, X. and Smirnova, I. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282 (1998) 2085-2088.
37. Quinn, M. R., Park, E. and Schuller-Levis, G. Taurine chloramine inhibits prostaglandin E2 production in activated RAW 264.7 cells by post-transcriptional effects on inducible cyclooxygenase expression. Immunol. Lett. 50 (1996) 185-188.
38. Reiling, N., Ulmer, A. J., Duchrow, M., Ernst, M., Flad, H. D. and Hauschildt. S. Nitric oxide synthase: mRNA expression of different isoforms in human monocytes/macrophages. Eur. J. Immunol. 8 (1994) 1941-1944.
39. Schneemann, M., Schoedon, G., Hofer, S., Blau, N., Guerrero, L. and Schaffner, A. Nitric oxide synthase is not a constituent of the antimicrobial armature of human mononuclear phagocytes. J. Infect. Dis. 167 (1993) 1358-1363.
40. Schuller-Levis, G. B., Gordon, R. E., Park, E., Pendino, K. J. and Laskin, D. L. Taurine protects rat bronchioles from acute ozone-induced lung inflammation and hyperplasia. Exp. Lung Res. 21 (1995) 877-888.
41. Schuller-Levis, G. B., Levis. W. R., Ammazzaloroso, M., Nostrati, A. and Park. E. Mycobacterial lipoarabinomannan induces nitric oxide and tumor necrosis factor alpha production in a macrophage cell line: downregulation by taurine chloramine. Infect. Immun. 62 (1994) 4671-4674.
42. Van Voorhis, W. C., Kaplan, G., Sarno, E. N., Horwitz, M. A., Steinman. R. M., Levis, W. R., Noguiera, N., Hair, L. S., Gattass, C. R. Arrick, B. A. and Cohn, Z. A. The cutanous infiltrates of leprosy-cellular characteristics and the predominant T-cell phenotypes. N. Engl. J. Med. 307 (1982) 1593-1597.
43. Vodovotz, Y., Bogdan, C., Paik. J., Xie, Q. W. and Nathan, C. Mechanisms of suppression of macrophage nitric oxide release by transforming growth factor beta. J. Exp. Med. 178 (1993) 605-613.
44. Weiss. S. J., Klein, R., Slivka, A. and Wei, M. Chlorination of taurine by human neutrophils: evidence for hypochlorous acid generation. J. Clin. Invest. 70 (1982) 598-607.
45. Zhang, X., Alley, E. W., Russel, S. and Morrison, D. C. Necessity and sufficiency of beta interferon for nitric oxide production in mouse peritoneal macrophages. Infect. Immun. 62 (1994) 33-40.
1. Ph.D.; Department of Immunology;
2. Ph.D., Department of Immunology;
3. Ph.D., Department of Developmental Biochemistry, New York State Institute for Basic Research in Developmental Disabilities, 1050 Forest Hill Road, Staten Island, New York 10314, U.S.A.
4. M.D., Staten Island University Hospital, Staten Island, New York 10305, U.S.A.
5. D.V.M., Ph.D., Department of Veterinary Medicine, Konkuk University, Seoul, Korea 143-701.
Reprint requests to Dr. Schuller-Levis at the above address or FAX 1-718-494-4884; email: georgia.schuller-levis@omr.state.ny.us
Received for publication on 29 February 2000.
Accepted for publication in revised form on 8 November 2000.