- Volume 68 , Number 4
- Page: 452–5
A Mycobacterium leprae isolate resistant to dapsone, Rifampin, Ofloxacin and Sparfloxacin
ABSTRACT
Mycobacterium leprae were isolated from a Japanese patient, and susceptibility to antileprosy drugs was examined by the mouse foot pad method. The isolate was susceptible to clofazimine and clarithromycin, and resistant to dapsone, rifampin, ofloxacin and sparfloxacin. Mutations were identified in the genes associated with resistance to these drugs. The risk of the emergence of leprosy with multidrug resistance is emphasized.RÉSUMÉ
Mycobacterium leprae fut isolé d'un patient japonais et la susceptibilité aux médicaments anti-lépreux furent évaluée en utilisant la méthode de la plante des pattes de souris. L'isolat était susceptible à la clofazimine et la clarithromycine, et résistante à la dapsone, la rifampicine, l'orofloxacine et la sparffoxacine. Des mutations furent identifiées parmi les gènes associés aux résistances à ces antibiotiques. Le risque de l'apparition de lèpres polychimio-résistantes est souligné.RESUMEN
Se aisló Mycobacterium leprae, de un paciente japonés. La susceptibilidad del aislado a las drogas antileprosas se estudió utilizando el modelo de la almohadilla plantar del ratón. El aislado fue susceptible a elofazimina y claritromicina, y resistente a dapsona, ri- fampina, olloxaeina y esparfloxacina. Se identificaron mutaciones en los genes asociados con la resistencia a estas drogas. Se subraya el riesgo de la emergencia de cepas multi-drogoresistentes.Since dapsone (DDS) was introduced for leprosy treatment, many effective antileprosy drugs have been added to develop an effective multidrug therapy (MDT) for leprosy. Prior to MDT for leprosy, clinically suspected DDS-resistant cases were reported (22). After identifying DDS-resistant strains of Mycobacterium leprae by the mouse foot pad method in 1964 (15), many primary or secondary drug-resistant cases have been reported not only for DDS but also for other drugs (4, 8, 14). Along with an increasing incidence of primary DDS resistance in some leprosy-endemic areas (3), M. leprae isolates with resistance to multiple drugs have been reported (2, 18). Because drug-resistant M. leprae have been identified among a significant number of relapse cases (6), it is important to consider that some cases not improving with treatment may harbor drug-resistant M. leprae. In this report we used the mouse foot pad method to examine the susceptibility to antileprosy drugs of an M. leprae strain isolated from a patient who maintained a high bacterial index (BI) for over 15 years.
MATERIALS AND METHODS
A biopsy specimen was obtained from a 51-year-old Japanese male first admitted to the National Leprosarium in 1963. The patient was treated with DDS monotherapy for 20 years at a dosage of 50 mg/day. Ri- fampin was added in 1982, and ofloxacin was also prescribed from 1993. His BI decreased to 1+ in 1979 but clinical disease recurred and the BI increased and continued between 5+ and 6+ after 1990. When he visited one of the authors in 1997, his clinical condition was aggravated and relapse occurred again. Resistance to antileprosy drugs was suspected since the patient's condition was not improving and because of the extended period of treatment with various antibiotics. Therefore, a biopsy sample was obtained from a new lepromatous lesion on the neck, and the bacteria were tested for susceptibility to antileprosy drugs.
The tissue specimen was processed to recover M. leprae by Nakamura's method with a slight modification (12). Briefly, the tissue was minced and homogenized with Hanks' balanced salt solution (HBSS) containing 0.05% Tween 80. The homogenate was centrifuged at 150 × g for 10 min, and the supernatant of the sample homogenate was treated with 0.05% trypsin at 37°C for 60 min. The suspension was centrifuged at 4000 × g for 20 min, and the sediment was resuspended in HBSS followed by treatment with 1% sodium hydroxide at 37°C for 15 min. The treated material was washed and resuspended in HBSS at the desired bacillary concentration.
Initial isolation of the bacilli was conducted by injecting nude mice since the viability of the bacilli in the sample treated with antileprosy drugs was uncertain. A bacillary suspension containing 1.0 × 106 in 0.05 ml was injected into the hind foot pads of BALB/c-nu/nu mice. Drug susceptibility tests were conducted with the bacilli recovered from the nude mice foot pads showing bacillary multiplication. Nude mouse-grown M. leprae (5 × 103 bacilli) were injected into each hind foot pad of inbred BALB/c mice; 54 mice were divided into 9 groups. The control group was fed standard pellet mouse chow MB 6E (Fun- abashi Nojyo, Japan). Eight experimental groups received diets mixed with DDS in concentrations of 0.0001%, 0.001% and 0.01% w/w of diet (10, 16), rifampin in concentration 0.01% (16), ofloxacin in concentration 0.15% (16), sparfioxacin in concentration 0.02% (19), clarithromycin in concentration 0.03% (5, 16), and clofazimine in concentration 0.001% (16). The concentration of rifampin was increased above the usual concentration (0.003%) since a preliminary study had shown rifampin at 0.003% did not completely inhibit the growth of bacilli in foot pads. Harvesting organisms from the foot pads was done 30 weeks after inoculation. Bacillary number in each foot pad was enumerated individually according to standard techniques (17).
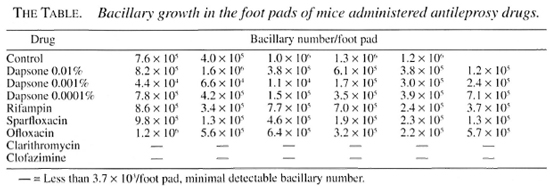
RESULTS
The results of the present study are given in The Table. Multiplication of M. leprae occurred in the foot pads of mice treated with DDS at 0.0001%, 0.001% and 0.01%, rifampin, ofloxacin and sparfioxacin. No bacillary growth was observed in the foot pads of mice fed the diets containing clarithromycin and clofazimine. The bacillary numbers in foot pads of three mice fed the chow containing 0.001% DDS were low and may be the result of technical error because bacillary growth in the mice fed the 0.01% DDS concentration was sufficient. The bacillary growth results of the control drug-susceptible isolate was completely suppressed by dietary administration of all drugs tested. These results showed that the patient isolate was resistant to DDS, rifampin, ofloxacin and sparfioxacin but susceptible to clarithromycin and clofazimine.
DISCUSSION
This is the third case of M. leprae resistant to multiple drugs. The first case of multidrug resistance to dapsone, rifampin and clofazimine in M. leprae was reported in 1996 (18) and the second case, resistant to dapsone, rifampin and ofloxacin, was reported in 1997 (2). The case presented in this report responded initially to DDS, with resistance appearing approximately 17 years after DDS monotherapy. The results suggest that the resistance to DDS is secondary resistance. The bacilli were also considered to be secondary rifampin resistant since the BI decreased from 6+ to 4+ after 5 years of treatment with rifampin. It seemed that DDS and rifampin, which were taken irregularly and at dosages lower than recommended, could have led to step-wise selection of the bacilli resistant to DDS and rifampin. There was almost no decrease in the BI after adding ofloxacin to the patient's treatment regimen. This suggested that the bacilli had already acquired resistance to quinolones before the administration of ofloxacin, although it has not been established whether the patient had received quinolone treatment at an earlier time.
Mutations of genes conferring resistance to DDS (11, 20), rifampin (7, 21), quinolones (1) and macrolides (9, 13) have been reported. Specific mutations in folP (Ile 53 forThr), rpoB (Leu 425 for Ser) and gyrA (Val 91 for Ala) have been shown but specific mutations have not been detected in 23S rRNA gene of this isolate (submitted). These mutations were concordant with the results of susceptibility testing in mouse foot pads. Because of accumulating evidence supporting the association of specific mutations and drug resistance, it seems reasonable to begin to exploit this information for the development of simple, diagnostic methods capable of determining drug resistance to these antileprosy drugs.
Early detection of drug-resistant bacilli among patients who are not improving clinically and better treatment with combinations of effective drugs must be considered to prevent further occurrence of resistance to current and new drugs for leprosy. An increase of leprosy cases with primary resistance to more than two drugs is a matter of concern and may threaten current strategies to control leprosy.
Acknowledgment. This study was supported by a grant for "Research on Emerging and Re-emerging Infectious Diseases" of the Japanese Ministry of Health and Welfare and a grant for "Research on International Collaboration in Medicine" of the International Medical Center, Japanese Ministry of Health and Welfare. We thank Dr. Thomas P. Gillis of the National Hansen's Disease Programs. Baton Rouge. Louisiana, U.S.A., for his excellent advice.
REFERENCES
1. Cambau, E. and Jarlier, V. Resistance to quinolones in mycobacteria. Res. Microbial. 147 (1996) 52-59.
2. Cambau, E., Perani, E., Guillemin, I., Jamet, P. and Ji, B. Multidrug-resistance to dapsone, rifampicin, and ofloxacin in Mycobacterium leprae. Lancet 349 (1997) 103-104.
3. dela Cruz, E., Cellona, R. V., Balagon, M. V. F., Villahermosa, L. G., Fajardo, T. T. Jr., Abalos, R. M., Tan, E. V. and Walsh, G. P Primary dapsone resistance in Cebu, The Philippines; cause for concern. Int. J. Lepr. 64 (1996) 253-256.
4. Diepen, T. W. Clofazimine-resistant leprosy, a case report. Int. J. Lepr. 50 (1982) 139-142.
5. Franzblau, S. G. and Hastings, R. C. In vitro and in vivo activities of macrolides against Mycobacterium leprae. Antimicrob. Agents Chemother. 32 (1988) 1758-1762.
6. Grosset, J. H., Guelpa-Lauras, C.-C., Bobin, P., Brucker, G., Cartel, J.-L., Constant-Desportes, M., Flageul, B., Frederic, M., Guillaume, J.-C. and Millan, J. Study of 39 documented relapses of multibacillary leprosy after treatment with rifampin. Int. J. Lepr. 57 (1989) 607-614.
7. Honore, N. and Cole, S. T. Molecular basis of rifampin resistance in Mycobacterium leprae. Antimicrob. Agents. Chemother. 37 (1993) 414-418.
8. Jacobson, R. R. and Hastings, R. C. Rifampin-resistant leprosy. (Letter) Lancet 2 (1976) 1304-1305.
9. Jamal, M. A., Maeda, S., Nakata, N., Kai, M., Fukuchi. K. and Kashiwabara, Y. Molecular basis of clarithromycin-resistance in Mycobacterium avium-intracellulare complex. Tuberc. Lung Dis. 80 (2000) 1-4.
10. Ji, B. Drug susceptibility testing of Mycobacterium leprae. Int. J. Lepr. 55 (1987) 830-835.
11. Kai, M., Matsuoka, M., Nakata, N., Maeda, S., Gidoh, M., Maeda, Y., Hashimoto, K., Kobayashi, K. and Kashiwabara, Y. Diamino-diphenylsulphone resistance of Mycobacterium leprae due to mutations in the dehydropteroate synthase gene. FEMS Microbiol. Lett. 177 (1999) 231-235.
12. Nakamura, M. [Elimination of contaminants in a homogenate of nude-mouse foot pad experimentally infected with Mycobacterium leprae.] Jpn. J. Lepr. 64 (1994) 47-50.
13. Nash, K. A. and Inderued, C. B. Genetic basis of macrolide resistance in Mycobacterium avium isolated from patients with disseminated diseases. Antimicrob. Agents Chemother. 39 (1995) 2625-2630.
14. Pearson, J. M. H., Rees, R. J. W. and Waters. M. F. R. Sulphone resistance in leprosy: a review of one hundred proven clinical cases. Lancet 2 (1977) 69-72.
15. Pettit, J. H. S. and Rees, R. J. W. Sulphone resistance in leprosy. An experimental and clinical study. Lancet 2 (1964) 673-674.
16. Rees, R. J. W. and Young, D. B. The microbiology of leprosy. In: Leprosy. 2nd edn. Hastings, R. C., ed. Edinburgh: Churchill Livingstone, 1994. pp. 49-83.
17. Shepard, C. C. and McRae, D. H. A method for counting acid-fast bacteria. Int. J. Lepr. 36 (1968) 78-82.
18. Shetty, V. P., Uplekar, M. W. and Antia, N. H. Primary resistance to single and multiple drugs in leprosy-a mouse foot pad study. Lepr. Rev. 67 (1996) 280-286.
19. Traore, I., Ji, B., Lienhardt, C., Bobin. P. and Grosset, J. Determination of the minimal effective dose of ofloxacin and sparfloxacin against M. leprae in the mouse foot pad system. Int. J. Lepr. 64 (1996) 142-145.
20. Williams. D. L., Spring, L., Harris, E., Roche, P. and Gillis, T. P. Dihydropteroate synthase of Mycobacterium leprae and dapsone resistance. Antimicrob. Agents Chemother. 44 (2000) 1530-1537.
21. Williams, D. L., Waguespack, C., Eisenach, K., Crawford, J. K., Portaels, F., Salfinger, M., Nolan, C. M., Abe, C., Stich-Groh, V. and Gillis, T. P. Characterization of rifampin resistance in pathogenic mycobacteria. Antimicrob. Agents Chemother. 38 (1994) 2380-2386.
22. Wolcott, R. R. and Ross, Sr. H. Exacerbation of leprosy during present day treatment. Int. J. Lepr. 21 (1953) 437-440.
1. Ph.D.; Leprosy Research Center, National Institute of Infectious Diseases, 4-2-1 Aobacho, Higashimurayama-shi, 189-0002 Tokyo, Japan.
2. Ph.D., Leprosy Research Center, National Institute of Infectious Diseases, 4-2-1 Aobacho, Higashimurayama-shi, 189-0002 Tokyo, Japan.
3. M.D., National Leprosarium Tama-Zensho-En, 4-2-1 Aobacho, Higashimurayama-shi, 189-8550 Tokyo, Japan.
Reprint requests to Dr. Matsuoka at the above address or FAX 81-42-394-9092; email: matsuoka@nih.go.jp
Received for publication on 5 October 2000.
Accepted for publication on 2 November 2000.