- Volume 68 , Number 4
- Page: 456–63
Use of ML dipstick as a tool to classify leprosy patients
ABSTRACT
Leprosy control services face the problem of leprosy patients being misclassified by the lack of or the poor quality of skin-smear examination services. Misclassification increases the risk of relapse due to insufficient treatment if a multibacillary (MB) patient is classified as paucibacillary (PB), thereby also prolonging the time that the patient is infectious. The World Health Organization (WHO) recommends at present an alternative classification based on the number of skin lesions. Its reliability, however, has been questioned. Our investigation sought to determine the usefulness of the ML Dipstick, a simple field assay to detect IgM antibodies to phenolic glycolipid-I of Mycobacterium leprae, for the classification of leprosy patients in addition to lesion count. In this study, 264 leprosy patients were investigated. Of 130 patients with a positive bacterial index (BI), 19 (14.6%) had less than 6 lesions and would have been classified as PB. Out of 134 patients with a negative BI, 26 (19.4%) had 6 or more lesions and would have been classified as MB patients if the lesion counting system would apply. Thus, the classification based on the number of lesions only was found to be 85% sensitive and 81% specific (using the BI as the gold standard) at detecting MB cases among the studied population. Sensitivity would have increased if patients would have been classified according to a combination of the number of lesions and the dipstick result. In that case patients are classified as MB when they are either dipstick positive (N = 16), have more than 6 lesions (N = 43), or both (N = 94). Patients negative for both dipstick and number of lesions would have been classified as PB (N = 111). The classification based on the number of lesions alone left 19 BI-positive cases classified as PB, while the combination method of the ML Dipstick and number of lesions left only 8 BI-positive cases classified as PB (5 borderline, 2 borderline lepromatous and 1 tuberculoid), thus preventing undertreatment. The combination method of the ML Dipstick and lesion counting was found to be 94% sensitive and 77% specific, which is an improvement of 9% (chi-squared test, p = 0.025) in sensitivity compared to lesion counting only. The results of this study indicate that testing all patients initially classified by lesion counting as PB (48% in our study population) with the dipstick can significantly contribute to improved classification of leprosy patients for treatment purposes.RÉSUMÉ
Les services de contrôle de la lèpre sont confrontés au problème de la mauvaise classification des patients lépreux par le manque ou la qualité moyenne des services faisant les examens de suc dermique. La classification dans use catégorie incorrecte augmente le risque de rechute causée par un traitement insuffisant d'un patient multibacillaire (MB) classifié comme paucibacillaire (PB), ce qui augmente également le temps pendant lequel un patient est infectieux. L'Organisation Mondiale de la Santé (OMS) recommande à présent une autre classification basée sur le nombre des lésions cutanées. Son efficacité a cependant été mise en doute. Le but de notre étude était de déterminer l'utilité de la bandelette ML, un test simple de terrain visant à détecter les IgM dirigés contre les glycolipides phénoliques de type I de Mycobacterium leprae, pour aider à la classification des patients lépreux en complément du nombre des lésions. Cette étude porte sur 264 patients lépreux. Parmi 130 patients ayant un index bactérioscopique (IB) positif, 19 (14.6%) présentaient moins de 6 lésions et auraient été faussement considérés comme des patients PB. Parmi 134 patients ayant un IB négatif, 26 (19,4%) présentaient 6 lésions ou plus et ils auraient été classifiés comme des patients MB si le système du dénombrement des lésions est appliqué. Ainsi, on trouve une sensibilité de 85% et une spécificité de 81 % de la méthode de classification basée uniquement sur le nombre de lésions, en utilisant l'IB comme méthode de référence, pour détecter Les patients multibacillaires dans la population étudiée. La sensibilité serait augmentée si les patients étaient classés à l'aide d'une combinaison du nombre de lésions et des résultats apportés par l'utilisation de la bandelette. Dans ce cas les patients sont classés MB s'ils présentent une bandelette positive (N = 16), ont plus de 6 lésions (N = 43) ou les deux (N = 94). Les patients négatifs à la fois pour la bandelette et le nombre de lésions, seraient classés comme PB (N = 111). La classification basée uniquement sur le nombre de lésions a laissé pour compte 19 cas ayant un IB positif classé comme PB, tandis que la méthode combinant la bandelette ML et le nombre de lésions n'a laissé pour compte que 8 cas ayant un IB positif classé comme PB (5 borderlines. 2 lépromateux borderlines et un tuberculoïde), limitant ainsi l'étendue des traitements insuffisants. La méthode combinant la bandelette ML et le décompte des lésions est 94% sensible et 77% spécifique, ce qui est line amélioration de 9% (p = 0.025 au test chi-square) de la sensibilité par rapport au seul décompte des lésions. Le résultat de cette étude indique une amélioration significative de la classification des patients hanséniens à visée thérapeutique si on teste tous les patients initialement classés comme PB par le seul décompte des lésions (48% de la population étudiée ici) avec la bandelette ML.RESUMEN
Los servicios de control de la lepra se encuentran con el problema de los pacientes mal clasificados debido a la carência o a la pobre calidad de las unidades de lectura de los extendidos de linfa cutánea. La clasificación errónea aumenta el riesgo de recaída porque un paciente multibacilar (MB) podría ser tratado como paucibacilar (PB), prolongando así el tiempo en el que el paciente es infeccioso. La Organización Mundial de la Salud (OMS) recomienda actualmente una clasificación alternativa basada en el número de lesiones en la piel, cuya confiabilidad ha sido cuestionada. Nuestra investigación estuvo orientada a determinar la utilidad de la prueba con la tira para PGL-I (un ensayo de campo simple para detectar anticuerpos IgM contra el glicolípido fenólico de Mycobacterium leprae) y el conteo de lesiones, en la clasificación de los pacientes. En el estudio se incluyeron 264 pacientes con lepra. De 130 pacientes con un indice bacteriano positivo (IB), 19 (14.6%) tuvieron menos de 6 lesiones y fueron clasificados como PB. De los 134 pacientes con un IB negativo. 26 (19.4%) tuvieron 6 o más lesiones y fueron clasfcados como MB según el sistema de cuenta de lesiones. La clasificación basada en el número de lesiones tuvo sólo una sensibilidad del 85% y una especificidad del 81% (usando el IB como estándar de oro) como sistema de detección de casos MB entre la población estudiada. La sensibilidad se habria incrementado si los pacientes se hubieran clasificado combinando los resultados de la cuenta de lesiones y de la tira para anticuerpos IgM. En ese caso, los pacientes serian clasificados como MB cuando son positivos por la tira (N = 16), cuando tienen más de 6 lesiones (N = 43), o cuando ocurren ambas cosas (N = 94). Los pacientes negativos en ambas pruebas habrían sido clasificados como PB (N = 111). La clasificación basada solamente en el número de lesiones dejó 19 casos con un IB positivo, clasficados como PB, mientras que la combinación de los métodos dejó sólo 8 casos IB positivos clasificados como PB (5 intermedios, BB; 2 lepromatosos subpolares y un tuberculoide, TT) previniendo así el tratamiento insuficiente del grupo de pacientes. La combinación de los métodos (cuenta de lesiones y la lira para anticuerpos anti-PGL-I) tuvo una sensibilidad del 94% y una especificidad del 77%, lo que representa una mejoría del 9% en sensibilidad (prueba de chicuadrada, p = 0.025), en comparación con la cuenta de lesiones sola. Los resultados de este estudio indican que se puede mejorar significativamente la clasificación de los pacientes (y su tratamiento), si todos los pacientes clasificados como PB por la cuenta de lesiones (48% en nuestra población de estúdio) se prueban también con la tira anti-PGL-I.Classification of leprosy patients into paucibacillary (PB) and multibacillary (MB) determines the duration of their treatment. MB patients are treated for a period of 24 months with a monthly supervised combination therapy consisting of rifampin, clofazamine and dapsone; whereas PB patients are treated for 6 months with rifampin and dapsone (1).
The methods used for leprosy classification have changed significantly over the years. In 1982 the World Health Organization (WHO) defined PB patients as those with indeterminate (I), tuberculoid (TT) or borderline tuberculoid (BT) leprosy with a bacterial index (BI) of less than 2+ at all sites; MB patients as those with a BI of 2+ or higher at any site (3). Later the definition was changed and patients with a positive BI at any site were classified as MB (24). However, as the results of skin smears were often of poor quality or not available at all (25), further simplification of the classification of patients has been introduced based on the number of body areas affected by skin and nerve lesions (23) and by an even simpler classification in some countries grouping patients with less than 6 skin lesions as PB patients and patients with 6 or more skin lesions as MB patients (26). However, the reliability of classification on clinical criteria only has been questioned, since a classification system based on lesion count only is prone to underestimation of the number of lesions, especially in areas of the world in which cultural factors may influence the physical examination of the patient. Underestimation of the number of lesions can lead to misclassification of MB patients as PB (11) and, thus, undertreatment.
The changes over the years in leprosy patient classification systems illustrate the difficulty of defining the border between PB and MB. Misclassification increases the risk of relapse due to insufficient treatment of an MB patient classified as PB, thereby increasing the time that the patient is infectious. Long before a relapse becomes manifest, leprosy patients may be highly infectious, transmitting the disease in the community.
Classification systems based either on bacteriological smear examination or on clinical findings have the need for well-trained leprosy workers in common, a possible limitation considering the integration of leprosy control into the general health service in many countries. In addition, both systems are liable to subjective interpretation. The currently practiced classification system based on clinical findings would benefit from a simple and robust test which gives results that are related to the bacterial load.
Several studies have shown that the presence of antibodies to the Mycobacterium leprae-specific phenolic glycolipid-I (PGL-I) correlates with the bacterial load of a leprosy patient (7, 12, 22). The large majority of PB patients are seronegative; whereas the large majority of MB patients are seropositive (2, 13, 15, 16, 21, 22). Studies monitoring the serum antibody levels to PGL-I during treatment further demonstrate that these levels correlate with the bacterial load: a decline during treatment corresponds with a declining BI (1, 12, 14, 16, 18). In addition, increasing levels of antibodies to PGL-I in patients have been associated with the development of relapses (9, 10). Detection of anti-PGL-I antibodies may thus be a useful tool to confirm the diagnosis of MB leprosy.
Recently, we have developed a dipstick that detects IgM antibodies to PGL-I that is suitable for field use. Studies demonstrate a high degree of agreement (97.2%) between the dipstick assay and the ELISA (4). The dipstick is a simple and rapid test, not dependent on specialized equipment, and employs highly stable reagents that do not require refrigeration.
Here we investigated the potential usefulness of the dipstick as a tool in classifying leprosy patients into PB or MB for treatment purposes.
MATERIALS AND METHODS
Study population. The population studied included 264 untreated leprosy patients attending the leprosy clinic at Oswaldo Cruz Foundation, Rio de Janeiro, Brazil, between January 1995 and May 1999. The FIOCRUZ Ethical Commission approved the study. All patients had given their consent to participate in the study.
Sera from patients were collected and kept frozen at -20°C. Patients were diagnosed based on clinical, bacteriologic and histopathological findings according to Ridley and Jopling (20), and the BI and the number of lesions were recorded. The BI was calculated as the mean BI of six skin smears. Clinical, bacteriologic and histopathological findings were recorded. For our study, we used the BI results to divide the patients into PB and MB, with a positive BI result at any site leading to classification as an MB patient.
The study population was composed of 16 indeterminate (I), 4 tuberculoid (TT), 102 borderline tuberculoid (BT), 47 midborderline (BB), 42 borderline lepromatous (BL), 43 lepromatous (LL) and 10 primary neuritic (PN) leprosy patients.
Dipstick assay. The dipstick assay for the detection of antibodies to PGL-I of M. leprae was prepared as described previously (4). The dipsticks have two bands: an antigen band consisting of the M. leprae-specific and immunodominant disaccharide epitope of PGL-I linked to bovine serum albumin via an octyl linker arm (ND-O-BSA) (8), and an internal control band consisting of anti-human IgM antibodies that bind IgM molecules from the serum. The IgM detection reagent consists of a lyophilized monoclonal anti-human IgM antibody linked with a colloidal dye. Briefly, dipsticks were wetted in distilled water for 15 sec and then incubated for 1 hr in a reaction vial containing 200 µl of the reconstituted detection reagent and 4 µl serum. At the end of the incubation period, the dipsticks were rinsed with tap water and air-dried at ambient temperature. A reddish-stained antigen band indicates a positive reaction. The results were scored as positive when staining was observed; no coloring (but with a positive control band) was scored as negative.
ELISA. The ELISA for the detection of IgM antibodies to PGL-I of M. leprae was performed essentially as described previously (3) using ND-O-BSA as the semi-synthetic analog of PGL-I. ND-O-BSA (0.0023 µg of sugar/ml) was diluted in a volatile ammonium acetate carbonate buffer (pH 8.2). Nunc-Immunoplates-II (Life Technologies, Taastrup, Denmark) were coated with 50 µl/well and left to dry at room temperature. As a control, 0.1 µg/ml bovine serum albumin (BSA) was used. Microtiter plates were blocked for 60 min with 100 µl of a 1% (w/v) BSA in PBS. After washing six times with PBS containing 0.1% (v/v) Tween-20 (PBST), the sera were diluted 1:300 in PBST containing 10% (v/v) normal goat serum (NGS) and 50 µl was added to each well. This was incubated at 37°C for 60 min and followed by another wash step. Peroxidase conjugated anti-human IgM conjugate (Capple/Organon Teknika, Turnhout, Belgium) was added (50 µl/well) at a 1:2000 dilution in PBST-10% NGS to the microtiter plate. After incubation at 37°C for 60 min, the washing procedure was repeated and 50 µl of the Sigma 3,3',5,5'-tetramethyl-benzidine (TMB) liquid substrate system was added to each well. In order to control for plate-to-plate and day-to-day variations, a positive reference serum was included in quadruplicate on each plate. The color reactions of the entire plate were stopped with 50 µl 2.5N H2SO4 when the optical density (OD) at 450 nm from the reference control serum reached an OD value of 0.6. All sera were tested in duplicate, and the ELISA results were expressed as the mean absorbance of the duplicates. The final OD value of each serum sample was calculated by subtracting the OD value of wells coated only with BSA from the OD value of the test wells coated with ND-O-BSA. The cut-off value for positivity was OD = 0.200 (5).
Data analysis. Data were analyzed using Epi-info version 6. The agreement between the dipstick, number of lesions and BI was determined by calculating kappa values with a 95% confidence interval (CI). Kappa values express the agreement beyond chance. Generally, a kappa value of 0.60 to 0.80 represents a substantial agreement beyond chance and a kappa value of >0.80 represents almost perfect agreement beyond chance.
RESULTS
Of the 264 patients, 134 were BI negative and classified as PB and 130 patients were BI positive and classified as MB.
Classification according to number of lesions. Table 1 shows how patients would have been classified based on counting the number of lesions: 137 as MB and 127 as PB patients. Out of 130 patients with a positive BI, therefore by definition MB patients, 19 (14.6%) had less than six lesions and would have been classified as PB using the lesion-counting system. Eighteen of them were treated with an MB regimen based on histopathological results. The one case treated with a PB regimen was a TT case with a negative dipstick result. Eleven were dipstick positive: 4 BB, 4 BL and 3 LL patients.
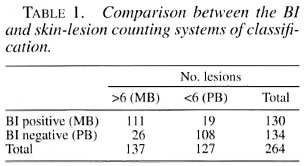
Out of 134 patients with a negative BI, therefore by definition PB patients, 26 (19.4%) had six or more lesions and would be classified as MB patients if the lesion-counting system would have been applied. Five were dipstick positive: 4 BT and 1 PN. Twenty-three were treated as PB patients. The three patients treated as MB (1 BT, 1 BB, 1 BL) were all dipstick negative. The sensitivity of the WHO system of classification based on the number of lesions for detecting MB cases in our study was 85% (95% CI 77.9-90.7), and the specificity was 81%. For our study population, the positive predictive value (PPV) was 81% and the negative predictive value (NPV) was 85%. The agreement between the bacteriological classification and the lesion-counting classification was 83.0%, kappa 0.66 (95% CI 0.54-0.78).
Classification according to ML Dipstick. Table 2 shows how patients would have been classified based on the presence of antibodies to PGL-I using the results of the ML Dipstick. One-hundred-ten would have been classified as MB and 154 as PB patients. From 130 patients with a positive BI (MB patients by definition), 30 (23.1%; 1 TT, 15 BB, 8 BL, 6 LL) were dipstick negative and would have been classified as PB if only the dipstick classification would have been applied.
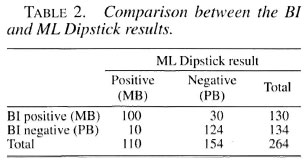
Out of 134 patients with a negative BI, therefore by definition PB patients, 10 (7.5%) had antibodies to PGL-I and would have been classified as MB patients according to dipstick results. They were all treated as PB. The mean skin BI from the dipstick-negative group was significantly lower than that of the dipstick-positive group [0.336 (S.D. = 0.994) versus 2.450 (S.D. = 1.518); p = 0.00005) as was the mean ELISA value [0.117 (S.D. = 0.250) versus 1.716 (S.D. = 1.164); p <0.0001].
The sensitivity of the ML Dipstick was 77% (95% CI 68.6-83.7) and the specificity 93%, with a PPV of 91% and an NPV of 81% in our study population. The agreement was 85%, kappa 0.7 (95% CI 0.59-0.83).
Classification combining ML Dipstick and number of lesions. Table 3 gives a detailed overview of the correlation between the BI classification on one hand and the results of the ML Dipstick and lesion counting on the other. Table 4 shows how patient classification systems based on either the BI or a combination of lesion counting and dipstick would compare. Patients who scored positive in dipstick only (N = 16), in number of lesions only (N = 43) and in both methods (N = 94) would all be classified as MB. Patients who were negative for dipstick and had less than six lesions would be classified as PB (N = 111). Eight patients would be missed using the combined method (1 TT, 5 BB, 2 BL). All were treated with an MB regimen, except for the TT case.
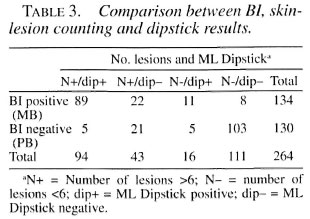
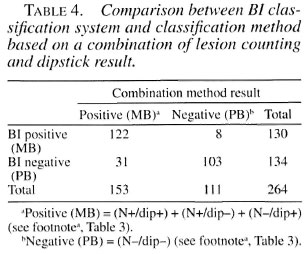
The sensitivity of the combination method was 94% (95% CI 87.8-97.1) and the specificity 77%. The PPV and NPV were 80% and 93%, respectively. The observed agreement was 85%, kappa 0.71 (95% CI 0.59-0.83).
DISCUSSION
For operational use, in the great majority of leprosy-endemic countries the classification of patients into PB and MB for treatment purposes is not based on the BI but determined on the basis of the number of lesions of the patient. Here, we have explored the possibility of using the detection of antibodies to PGL-I through a simple dipstick assay as a marker for the bacterial load of a patient and, consequently, as an additional tool for the classification into PB and MB leprosy. We showed that the system of clinical classification based on lesion counting in combination with the dipstick generated both a higher sensitivity for detecting MB patients and an increased ability to correctly identify PB cases.
Classification of patients is subjective and, therefore, it is difficult to get consistency under field conditions. Even in an ideal setting there will still be a small but significant number of BI-positive, therefore MB, cases being treated with a PB regimen. Croft and co-workers (11) reported a sensitivity of 89% for the WHO system of classification based on counting lesions. In our study we found a somewhat lower sensitivity of 85%, meaning that 15% of the MB patients would be incorrectly treated as PB. It is known that MB patients receiving PB treatment due to misclassification are susceptible to develop a relapse (11).
When comparing the dipstick and BI methods, the 85% agreement shows that the seropositivity found corresponded well with the BI of patients. Others have reported comparable results (7, 12, 22). Since antibodies to PGL-I are thought to reflect the bacterial load of the host (1, 12, 14, 16, 17), it is likely that the dipstick-negative and BI-pos- itive patients have less bacteria in their bodies than the BI of the skin suggests, but it is impossible to assess whether these patients are being overtreated. We have seen in our previous study that BI-positive patients, therefore MB, who did not present antibodies to PGL-I did not relapse, although they had received a short course of treatment. In contrast, all relapsed MB cases were seropositive by the dipstick method (6).
It has been shown before that patients may have a low or negative BI in their skin while bacteria may be found in the deeper tissue and in the nerves (6, 19). Since antibodies to PGL-I are a reflection of the total bacterial load in the body, again we could argue that the 10 PB patients with a positive dipstick result may have a higher bacterial load and, therefore, should have received longer treatment. False-positive classification into MB and consequent overtreat- ment, although slightly affecting the cost- effectiveness of the control program, can nevertheless be favored over false-negativity and undertreatment since that would result in a less effective control program due to the emergence of relapses (6, 23).
As described above, we found a sensitivity of 85% for the WHO system of classification according to the number of lesions. When combining the two methods, dipstick and lesion counting, we found a higher sensitivity (94%) to detect true MB cases and the chance of classifying a PB patient correctly was 93%. Still, eight BI-positive MB patients (6%) would be missed and treated as PB. Although they presented with low antibody levels, it was not possible to assess if 6 months of therapy had been sufficient. For this, a trial should be performed in which treatment is co-determined by the dipstick result. Previous studies indicate that antibody detection can be used for classification of leprosy patients (6, 22). The use of antibody detection as an additional tool for leprosy classification may be the key to decreasing the significant numbers of MB patients suspected to be undertreated by the lesion-counting system. The results of this study suggest that testing all patients initially classified as PB (48% in our study population) with the dipstick assay would be very helpful in this respect.
A combination of both methods showed a significant decrease in misclassification compared to the lesion counting only. We report an improvement of 9% (chi squared test, p = 0.025) in the sensitivity; therefore the combined method is a useful tool for the classification of leprosy patients under field conditions. The dipstick method is simple, can be performed using either serum or finger-prick blood (5), and is easy for health workers to use.
Acknowledgment. This study was financially supported by the Netherlands Leprosy Relief and the Scientific Research for the Tropics (WOTRO) fund of NWO (Nederlandse Organisatie voor Wetenschap- pelijk Onderzoek). ND-O-BSA (Contract NOl AI 55262, to CSU. PJB, PI) was kindly provided by Dr. D. Chatterjee, Colorado University, Denver, Colorado, U.S.A. We thank Dr. Elizabeth Pereira Sampaio for her support and help during the research.
REFERENCES
1. Bach, M. A., Wallach, D., Flageul, B., Hoffenbach, A. and Cottenot, F. Antibodies to phenolic glycolipid-l and to whole Mycobacterium leprae in leprosy patients: evolution during therapy. Int. J. Lepr. 54 (1986) 256-267.
2. Brett, S. J., Draper, P., Payne, S. N. and Rees, R. J. Serological activity of a characteristic phenolic glycolipid from Mycobacterium leprae in sera from patients with leprosy and tuberculosis. Clin. Exp. Immunol. 52 (1983) 271-279.
3. Brett, S. J., Payne, S. N., Gigg, J., Burgess, P. and Gigg, R. Use of synthetic glycoconjugates containing the Mycobacterium leprae specific and immunodominant epitope of phenolic glycolipid-I in the serology of leprosy. Clin. Exp. Immunol. 64 (1986) 476-483.
4. Buhrer, S. S., Smits, H. L., Gussenhoven, G. C., van Ingen, C. W. and Klatser, P. R. A simple dipstick assay for the detection of antibodies to phenolic glycolipid-I of Mycobacterium leprae. Am. J. Trop. Med. Hyg. 58 (1998) 133-136.
5. Buhrer-Sekula, S., Cunha. M. G., Ferreira, W. A. and Klatser, P. R. The use of whole blood in a dipstick assay for detection of antibodies to Mycobacterium leprae: a field evaluation. FEMS Immunol. Med. Microbiol. 21 (1998) 197-201.
6. Buhrer-Sekula, S., Cunha, M. G. S., Foss, N. T., Oskam, L., Faber. W. R., and Klatser, P. R. Identification of leprosy patients who have an increased risk of relapse using a simple dipstick assay (submitted for publication).
7. Cellona, R. V., Walsh, G. P., Fajardo, T. T., Jr., Abalos, R. M., dela Cruz, E. C., Guido-Villahermosa, L., Felicio-Balagon, M. V., Steenbergen, G. J. and Douglas, J. T. Cross-sectional assessment of ELISA reactivity in leprosy patients, contacts, and normal population using the semisynthetic antigen natural disaccharide octyl bovine serum albumin (ND-O-BSA) in Cebu, The Philippines. Int. J. Lepr. 61 (1993) 192-198.
8. Chatterjee, D., Cho, S.-N., Brennan, P. J. and Aspinall, G. O. Chemical synthesis and seroreac- tivity of O-(3,6-di-O-methyl-beta-D-glucopyranosyl)-( 1- 4)-O-(2.3-di-O-me). Carbohydr. Res. 156 (1986) 39-56.
9. Chin-A-Lien, R. A., Faber, W. R., van Rens, M. M., Leiker, D. L., Naafs, B. and Klatser, P. R. Follow-up of multibacillary leprosy patients using a phenolic glycolipid-I-based ELISA. Do increasing ELISA-values after discontinuation of treatment indicate relapse? Lepr. Rev. 63 (1992) 21-27.
10. Cho, S.-N., Cellona, R. V., Fajardo, T. T., Jr., Abalos, R. M., dela Cruz, E. C., Walsh, G. P., Kim, J. D. and Brennan, P. J. Detection of phenolic glycolipid-I antigen and antibody in sera from new and relapsed lepromatous patients treated with various drug regimens. Int. J. Lepr. 59 (1991) 25-31.
11. Croft, R. P., Smith, W. C., Nicholls, P. and Richardus, J. H. Sensitivity and specificity of methods of classification of leprosy without use of skin-smear examination. Int. J. Lepr. 66 (1998) 445-450.
12. Douglas, J. T., Steven, L. M., Fajardo, T., Cellona, R. V., Madarang, M. G., Abalos, R. M. and Steenbergen, G. J. The effects of chemotherapy on antibody levels in lepromatous patients. Lepr. Rev. 59 (1988) 127-135.
13. Douglas, J. T. and Worth, R. M. Field evaluation of an ELISA to detect antibody in leprosy patients and their contacts. Int. J. Lepr. 52 (1984) 26-33.
14. Gelber, R. H., Li, F., Cho, S.-N., Byrd, S., Rajagopalan, K. and Brennan, P. J. Serum antibodies to defined carbohydrate antigens during the course of treated leprosy. Int. J. Lepr. 57 (1989) 744-751.
15. Hussain, R., Jamil, S., Kifayet, A., Firdausi, F., Dockrell, H. M., Lucas, S. and Hasan, R. Quantitation of IgM antibodies to the M. leprae synthetic disaccharide can predict early bacterial multiplication in leprosy. Int. J. Lepr. 58 (1990) 491-502.
16. Klatser, P. R., de Wit, M. Y., Fajardo, T. T., Cellona. R. V., Abalos, R. M., dela Cruz, E. C., Madarang, M. G., Hirsch, D. S. and Douglas, J. T. Evaluation of Mycobacterium leprae antigens in the monitoring of a dapsone-based chemotherapy of previously untreated lepromatous patients in Cebu, Philippines. Lepr. Rev. 60 (1989) 178-186.
17. Meeker, H. C., Levis, W. R., Sersen, E., Schuller-Levis, G., Brennan, P. J. and Buchanan, T. M. ELISA detection of IgM antibodies against phenolic glycolipid-I in the management of leprosy: a comparison between laboratories. Int. J. Lepr. 54 (1986) 530-539.
18. Meeker, H. C., Schuller-Levis, G., Fusco, F., Giardina-Becket, M. A., Sersen. E. and Levis, W. R. Sequential monitoring of leprosy patients with serum antibody levels to phenolic glycol- ipid-1, a synthetic analog of phenolic glycolipid-I, and mycobacterial lipoarabinomannan. Int. J. Lepr. 58(1990) 503-511.
19. Ponnighaus, J. M., Lienhardt, C., Lucas, S., Fine, P. E. and Sterne, J. A. Comparison of bacillary indexes in slit-skin smears, skin and nerve biopsies; a study from Malawi. Int. J. Lepr. 65 (1997) 211-216.
20. Ridley, D. S. and Jopling, W. H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34 (1966) 255-273.
21. Roche, P. W., Britton, W. J., Failbus, S. S., Neupane, K. D. and Theuvenet, W. J. Serological monitoring of the response to chemotherapy in leprosy patients. Int. J. Lepr. 61 (1993) 35-43.
22. Roche, P. W., Britton, W. J., Failbus, S. S., Williams, D., Pradhan, H. M. and Theuvenet, W. J. Operational value of serological measurements in multibacillary leprosy patients: clinical and bacteriological correlates of antibody responses. Int. J. Lepr. 58 (1990) 480-490.
23. van Brakel, W. H., de Soldenhoff, R. and McDougall, A. C. The allocation of leprosy patients into paucibacillary and multibacillary groups for multidrug therapy, taking into account the number of body areas affected by skin, or skin and nerve lesions [see comments]. Lepr. Rev. 63 (1992) 231-246.
24. WHO Expert Committee on Leprosy. Sixth report. Geneva: World Health Organization. 1988. Tech. Rep. Ser. 768.
25. WHO Expert Committee on Leprosy. Seventh report. Geneva: World Health Organization. 1998. Tech. Rep. Ser. 874.
26. WHO Study Group. Chemotherapy of leprosy. Geneva: World Health Organization. 1994. Tech. Rep. Ser. 847.
1. M.Sc.; Department of Biomedical Research, Royal Tropical Institute (KIT). Meibergdreef 39, 1105 AZ Amsterdam, The Netherlands.
2. Ph.D.; Department of Biomedical Research, Royal Tropical Institute (KIT). Meibergdreef 39, 1105 AZ Amsterdam, The Netherlands.
3. Ph.D., Department of Biomedical Research, Royal Tropical Institute (KIT). Meibergdreef 39, 1105 AZ Amsterdam, The Netherlands.
4. M.D., Ph.D.; Department of Leprosy Research. Oswaldo Cruz Institute (FIOCRUZ), Av. Brasil 4365. Manguinhos, Rio de Janeiro, Brazil.
5. M.D., Ms.C.; Department of Leprosy Research. Oswaldo Cruz Institute (FIOCRUZ), Av. Brasil 4365. Manguinhos, Rio de Janeiro, Brazil.
6. M.D., Ph.D.; Department of Leprosy Research. Oswaldo Cruz Institute (FIOCRUZ), Av. Brasil 4365. Manguinhos, Rio de Janeiro, Brazil.
7. M.D., Ph.D., Department of Leprosy Research. Oswaldo Cruz Institute (FIOCRUZ), Av. Brasil 4365. Manguinhos, Rio de Janeiro, Brazil.
8. B.Sc.; Department of Dermatology, Academic Medical Center, University of Amsterdam, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands.
9. B.Sc.; Department of Dermatology, Academic Medical Center, University of Amsterdam, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands.
10. M.D., Ph.D., Department of Dermatology, Academic Medical Center, University of Amsterdam, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands.
Reprint requests to Samira Buhrer-Sekula at the above address or FAX 31-20-697-1841; e-mail: s.buhrer@kit.nl
Received for publication on 16 June 2000.
Accepted for publication in revised form on 28 September 2000.