- Volume 68 , Number 4
- Page: 405–9
Long-term follow up of multibacillary leprosy patients with high bi treated with WHO/MDT regimen for a fixed duration of two years
ABSTRACT
Forty-six, newly detected, previously untreated multibacillary (MB) patients with a bacterial index (BI) of >3+ who had received WHO/MDT for 2 years were followed up for a total duration of 424 person-years and a mean duration of 9.26 ± 2.98 years per patient. The BIs of the patients continued to fall, and all of the patients, except one, reached skin-smear negativity. WHO/MDT was well accepted and well tolerated. Relapse, which was defined as an increase in the BI of 1+ or more with or without clinical evidence of activity, was observed in only one patient, giving a relapse rate of 2.2% or 0.23 per 100 person-years in patients with a BI of >3+ after long-term follow up. This patient was started on a second course of WHO/MDT to which he responded favorably. WHO/ MDT for a fixed duration of 2 years for MB patients as recommended by the WHO is vindicated.RÉSUMÉ
Quarante six patients multibacillaire (MB) nouvellement détectés et non traités auparavant, avec un index bactérioscopique (IB) supérieur ou égal à 3+ et qui ont reçu une PCT/OMS pendant 2 années furent suivies pendant une durée totale de 424 personnes-années et une durée moyenne de 9,26 ± 2,98 années par patient. L'IB des patients continua à décroître et tous les patients, à l'exception d'un seul, atteignirent un statut négatif à l'examen du suc dermique. La PCT/OMS fut bien acceptée et bien tolérée. La rechute, quî fut définie comme une augmentation de l'IB de 1+ ou plus avec ou sans évidence clinique d'activité de la maladîe, ne fut observée que chez un patient, ce qui correspond à un taux de rechute de 2,2%, soit 0,23 pour 100 personnes-années chez les patients avec un IB de >3+ après suivi à long terme. Ce patient reçut un second traitement PCT/OMS avec une réponse favorable. Ces résultats défendent l'intérét et l'usage de la PCT/OMS à durée fixe de 2 ans pour les patients MB.RESUMEN
Se hizo cl seguimiento, por un total de 424 persona-años y una duración media de 9.26 ± 2.98 anos por paciente, de 46 pacientes con lepra multibacilar recién detectada, que recibieron la poliquimioterapia recomendada por la Organización Mundial de la Salud (PQT/OMS) durante 2 años. Los indices bacteriológicos (IB) de los pacientes continuaron decayendo y todos los pacientes, excepto uno, llegaron a ser negativos en los exámenes de linfa cutánea. La PQT/OMS fue bien aceptada y bien tolerada. La recaída, definida como un aumento en el IB de 1+ o más, con o sin evidencias de actividad clínica, se observó en un solo paciente, dando una tasa de recaída de 2.2% o de 0.23 por 100 persona-años en pacientes con un IB de >3+, después de un tiempo largo de seguimiento. Este paciente fue sometido a un segundo ciclo de tratamiento con PQT/OMS, al cual respondió favorablemente. Los resultados corroboran la eficacia del tratamiento de los pacientes con lepra MB con la PQT recomendada por Ia OMS durante un tempo fijo de 2 años.The World Health Organization (WHO), to overcome the problem of drug resistance and to introduce an effective and practicable regimen of finite duration, in 1982 recommended multidrug therapy (WHO/MDT) for the treatment of leprosy (12). Multibacillary (MB) leprosy was 'to be treated by daily dapsone and clofazimine together with monthly rifampin plus a supplemental higher dose of clofazimine for at least 2 years and was to be continued, whenever possible, until skin smears became negative. Since the data on limiting WHO/MDT in MB leprosy to 2 years rather than continuing until skin-smear negativity were favorable, the WHO Study Group in 1994 recommended that all MB patients be given the standard WHO/MDT regimen for 24 months (FDT) (10).
Doubts have been expressed that 2 years of WHO/MDT may not be adequate in patients with a high bacterial index (BI). In the THELEP trials in Chingleput and Bamako, where thymectomized-irradiated (TR) mice were used, persisting Mycobacterium leprae were detected in 9% of the borderline (BL) and lepromatous (LL) patients with a high initial BI after completion of 2 years of WHO/MDT (5). It is feared that persisting M. leprae would cause relapse in a large proportion of patients after WHO/MDT is withdrawn (6).
Published data from most of the centers have shown that FDT is very effective (9). The overall relapse rate is less than 1% (11). However, the duration of follow up in most of the patients has been relatively short; whereas relapse is expected 5 ± 2 years after stopping MDT (2, 4). The Marchoux Chemotherapy Study Group reported a relapse rate of 20% in patients with a high initial BI after a mean follow up of 72.7 ± 17.3 months per patient. All of the patients who relapsed had an initial BI of 4+ or more (2). This study was undertaken to assess the long-term efficacy of WHO/MDT of a fixed duration of 2 years in the treatment of leprosy patients with a BI of 3+ or more.
MATERIALS AND METHODS
The Schieffelin Leprosy Research and Training Centre, Karigiri (SLR&TC), India, has carried out leprosy control activities in Gudiyatham Taluk since 1962. Until 1980 all patients were treated with dapsone monotherapy. In 1981 WHO/MDT was introduced in Gudiyatham Taluk, and all MB patients were started on MDT for a minimum period of 2 years or until skin-smear negativity, whichever occurred later. Since 1984 MB patients have been treated with FDT. From January 1984 to May 1993, 65 newly detected, previously untreated MB patients with a BI of 3+ or more were started on FDT.
While on WHO/MDT they were reviewed monthly to assess clinical status and complications. The intake of dapsone was monitored using the "tablet count" and "urine dapsone/creatinine ratio." After release from treatment (RFT) the patients were seen once every 3 months. Detailed clinical assessment was done and a skin smear was taken yearly. From 1996 onward these patients have not been seen regularly. Whenever they attended the clinic a detailed clinical examination with reference to leprosy was carried out and skin smears were taken, if no such examination had been performed within the last year.
Of the 65 patients, 57 successfully completed the scheduled treatment, 4 patients migrated, 2 refused treatment and 2 died. These 57 patients were eligible for further follow up. A detailed clinical examination was carried out, nerve function impairments were assessed using standard methods (7), and skin smears from routine as well as selective sites were taken. Relapse was defined as an increase in the mean BI of 1+ or more with or without clinical signs of activity.
RESULTS
Of the 57 patients eligible for reassessment, 46 could be reevaluated because 5 patients had died and 6 had migrated. The mean age of these 46 patients at the time of induction into the study was 35.1 ± 14.96 years. Thirty-three (71.7%) were males and 13 (28.3%) females. Twenty-six (56.5%) were classified as BL and 20 (43.5%) as LL. While all of the patients had an initial mean BI of 3+ or more, in 17 (37%) patients it was 4+ or more. The mean of the average BIs before starting treating was 3.79+ ± 0.55+. Seven patients had a BI >3+ at RFT.
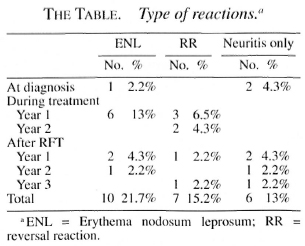
During treatment 14 (30.5%) patients developed reactions; 6 (13%) had erythema nodosum leprosum (ENL), 5 (10.8%) reversal reaction (RR) and 3 (6.5%) neuritis only. The Table shows the episodes of RR, ENL and neuritis only and the time of their occurrence in relation to treatment. Nine (19.5%) patients developed post-treatment reactions during surveillance; 2 (4.3%) had ENL, 3 (6.5%) RR and 4 (8.6%) neuritis only. Reactions occurred up to 3 years after RFT, and all reactions were treated with steroids.
Two patients developed adverse drug reaction to dapsone; one developed dapsone syndrome and the other only a cutaneous reaction. In both of these patients WHO/MDT was continued without dapsone. Although clofazimine discoloration was seen in all patients, it did not pose any problem of compliance since the patients had been educated about it. A female leprosy patient delivered a healthy female child while on WHO/MDT. The child had clofazimine discoloration.
While all of the patients were followed up for a minimum duration of 5 years after RFT, 35 (75.6%) patients were followed up for 7 years or more. By the end of March 2000, the total duration of follow up of the patients after RFT was 424 person-years and the mean duration of follow up was 9.3 ± 2.98 years per patient.
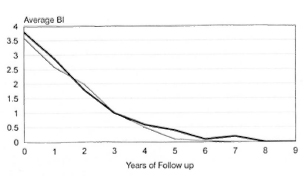
The Figure. Fall in BIs of patients on FDT and MDT until smear negativity. - = FDT; - = MDT until smear negativity.
The average clinical attendance during the treatment period was 89.5%, ranging between 61.5% and 100%. While on treatment the tablet count tallied with the expected number, and urinary dapsone/creatinine ratios remained positive in all of the patients, whenever tested, thus confirming regular intake of the drugs.
The BIs of the patients continued to decline after RFT and all patients, except one, reached skin-smear negativity. The Figure shows the fall of the mean BI in the patients treated with FDT and is compared with the fall in BI in a group of MB patients who received WHO/MDT until skin-smear negativity. The fall in BIs in both the groups was similar. The patient in whom the skin smear remained positive without reaching negativity was a BL case whose BI before starting treatment and at RFT was 4.4+ and 4+, respectively. After RFT his BI continued to fall. Eight years after RFT, when the patient was last seen, his BI was 0.5+. He was clinically inactive, and there was no evidence to suggest relapse. On the average, patients with an initial BI between 3+ and 3.9+ reached skin-smear negativity 2.37 ± 1.61 years after RFT, and patients with an initial BI >4 reached smear negativity 3.57 ±1.81 years after RFT.
Relapse was observed in only one male patient who was 39 years old at the time of registration. His BI initially and at RFT was 3+ and 2+, respectively. Three years after RFT he became skin-smear negative. Thirteen years after RFT, and after remaining smear negative for years, he was seen with multiple erythematous, infiltrated, hypes- thetic to nonanesthetic macules and plaques on the trunk and extremities. His skin smears showed a mean BI of 2.5+. A diagnosis of relapse as BL leprosy was made. A biopsy from a lesion on the back confirmed the clinical diagnosis, and he was restarted on WHO/MDT. After three pulses of WHO/MDT although he was clinically still active, his skin smear had declined to 2+.
DISCUSSION
The relapse rate provides the ultimate proof of successful treatment of any infectious disease. The duration of follow up required in leprosy after completion of WHO/MDT to assess the relapse rate accurately is not yet known. According to some studies, relapse in leprosy occurs late, usually more than 5 years after RFT (2, 4, 8) and, therefore, it is desirable that patients should be followed for 7-10 years after completion of treatment (3).
In the present study, 46 newly detected, previously untreated Mb patients with a BI of >3+, who had received WHO/MDT for a fixed duration of 2 years, were followed up for a minimum duration of 5 years and a mean duration of 9.3 ± 3.0 years after completion of WHO/MDT. Only one relapse was seen. WHO/MDT was well accepted and well tolerated by all of the patients. Clofazimine discoloration was seen in all the patients but it did not pose any problem with compliance. Complications related to WHO/MDT were minimal.
The Marchoux Chemotherapy Study Group followed up a group of 35 MB patients with a mean BI of 3.8+ who were treated with WHO/MDT for 2 years. After a follow-up period of 41.9 ± 12.1 months, they reported a single relapse, giving a relapse rate of 2.9% or 0.8 per 100 person-years (4). Two and a half years later, after a mean follow up of 72.7 ± 17.3 months, seven patients had relapsed. Thus, the overall relapse rate was 20% or 3.3 per 100 patient-years (2). Four of the seven patients had relapsed in years 6-7 of follow up and one in year 8. The mean time of relapse was 62.7 ± 18.7 months. In all of the seven patients who relapsed, the pretreatment BI was >4+, and five of them had a BI of >3+ at RFT. The Marchoux Chemotherapy Study Group concluded that two factors govern the risk of relapse: 1) high bacterial load in the patients and 2) long period of follow up after completion of treatment.
There are very few other studies in which patients who have been treated with FDT have been followed up for more than 5 years after RFT. In a questionnaire survey carried out by WHO, 20,141 MB patients, of whom 1414 were followed until year 9 after completion of WHO/MDT, 67 patients were reported to have relapsed. The cumulative risk of relapse was 0.77%. The relapse rates in years 6 and 7 were similar to those of years 3 and 4 (11).
At SLR&TC, Karigiri, India, 261 MB patients treated with FDT were followed up for a mean duration of 6.14 ± 2.11 years per patient, a total duration of 886 person-years after RFT. No relapse was seen (8). Li, et al. (3) from China reported a relapse rate of 0.19/1000 in 5981 MB patients, of whom 3068 were followed up for more than 5 years after completion of FDT. Out of a total of 5 relapses 1 had relapsed during year 4 and 2 each had relapsed during years 5 and 7 of follow up (3). Dasananjali, et al. reported no relapse in 51 MB patients who were followed up for 2 to 10 years with a mean duration of 8 years per patient (1).
In our study the mean BI of patients was 3.79+ ± 0.55+. While all the patients had an initial BI of >3+, 17 (37%) had a BI of >4+. The mean duration of follow up after RFT was 9.26 ± 2.98 years per patient with the total duration of follow up being 424 person-years. Only one patient whose BI initially and at RFT was 3+ and 2+, respectively, relapsed, giving a relapse rate of 2.17% or a risk of relapse of 0.23 per 100 person-years in patients with a BI of >3+ after a prolonged follow up. However, since the patient belongs to a leprosy-endemic area and relapsed 13 years after RFT, reinfection with M. leprae is also a possibility.
In conclusion, WHO/MDT for a fixed duration of 2 years is very effective, safe and well tolerated in MB patients. Since the risk of relapse is very low, even in patients with a high BI, there is no need for active post-MDT surveillance for the purpose of detecting relapse. Instead, we recommend that patients should be educated to report as soon as they notice any lesion suspicious of leprosy. The few relapses that occur can be retreated with a second course of WHO/MDT.
Acknowledgment. We are thankful to Professor Charles K. Job, Consultant Pathologist, St. Thomas Hospital and Leprosy Centre, Chettupattu, India; Dr. P. S. S. Sundar Rao, Director; Dr. S. Arunthathi, Head, Department of Medicine and Mrs. Nisha Kurein, Bio- statistician, SLR&TC, Karigiri, India, for their encouragement and guidance. We are also grateful to Mr. Y. Nallathambi, Physiotherapy Technician; Mr. S. Vincent, Laboratory Technician; Mr. P. Dorairaj, Records Technician, for their technical assistance and to Mr. C. Lewis Kumar for secretarial assistance. "The Field Trials of Fixed Duration Chemotherapy in MB Leprosy" received financial support from the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR).
REFERENCES
1. Dasananjali, K., Schreuder, P. A. M. and Pirayavaraporn, C. A study on the effectiveness and safety of the WHO/MDT regimen in the northeast of Thailand: a prospective study 1984-1996. Int. J. Lepr. 65(1997)28-36.
2. Jamet, P., Ji, B. and the Marchoux Chemotherapy Study Group. Relapses after long-term follow up of multibacillary patients treated by WHO multibacillary regimen. Int. J. Lepr. 63(1995)195-201.
3. Li. H., Hu, L., Huang, W., Liu, G., Yuan, I., Jin, Z., Li, X., Li, J. and Yang, Z. Risk of relapse in leprosy after fixed-duration multidrug therapy. Int. J. Lepr. 65(1997)238-245.
4. Marchoux Chemotherapy Study Group. Relapses in multibacillary leprosy patients after stopping treatment with rifampin-containing combined regimens. Int. J. Lepr. 60(1992)525-535.
5. Subcommittee on Clinical Trial of the Chemotherapy of Leprosy (THELEP) Scientific Working Group of the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases. Persisting Mycobacterium leprae among THELEP trial patients in Bamako and Chingleput. Lepr. Rev. 58(1987)7-16.
6. Subcommittee on Clinical Trial of the Chemotherapy of Leprosy (THELEP) Scientific Working Group of the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases. The THELEP controlled clinical drug trials. Int. Lepr. 55 Suppl. (1987) 864-871.
7. van Brakel, W. H. and Khawas, I. B. Nerve damage in leprosy: an epidemiological and clinical study of 396 patients in West Nepal-Part 1. Definitions, methods and fequencies. Lepr. Rev. 65 (1994)204-221.
8. Vijayakumaran, P., Jesudasan, K. and Manimozhi, N. Fixed-duration therapy (FDT) in multibacillary leprosy: efficacy and complications. Int. J. Lepr. 64(1996)123-127.
9. WHO Expert Committee on Leprosy. Seventh report. Geneva: World Health Organization. 1998. Tech. Rep. Ser. 874.
10. World Health Organization. Progress towards the elimination of leprosy as a public health problem. Wkly. Epidemiol. Rec. 68(1993)181-188.
11. World Health Organization. The Leprosy Unit, Division of Tropical Diseases. Risk of relapse in leprosy. Geneva: World Health Organization, 1994. WHO/CTD/LEP/94.1.
12. WHO Study Group. Chemotherapy of leprosy for control programmes. Geneva: World Health Organization, 1982. Tech. Rep. Ser. 675.
1. M.B.B.S., M.D., Specialist, Head, Department of Community Health.
2. M.B.B.S., D.H.E., Specialist; Department of Community Health.
3. M.B.B.S., D.N.B., Head, Department of Community Health.
4. M.B.B.S., D.T.P.H., Ph.D. (deceased), Epidemiologist; Department of Epidemiology and Leprosy Control.
5. M.B.B.S., D.T.M.&H., Dip. Epid. (deceased), Epidemiologist, Department of Epidemiology and Leprosy Control.
6. M.Sc., Biostatistician, Department of Biostatistics, Schieffelin Leprosy Research and Training Center, Karigiri, Vellore District, Tamil Nadu 632 106, India.
Reprint requests to Dr. Shaw at the above address or FAX 91-416-274274.
Received for publication on 28 August 2000.
Accepted for publication on 15 November 2000.
* Means throughout are means ± S.D.