- Volume 68 , Number 4
- Page: 410–6
Frequency and extent of thickening of the nucleated epidermis in leprosy lesions
ABSTRACT
Using 28 specimens of clinically normal skin from lepromatous leprosy subjects as a standard for comparison, the mean thickness of the nucleated epidermis was found to be significantly increased in untreated lesions from 16 borderline tuberculoid, 21 erythema nodosum leprosum (ENL), and 14 reversal reaction patients, but was unchanged in borderline lepromatous and lepromatous patients. Using specimens from 36 untreated lepromatous and borderline lepromatous lesions as the standard for comparison with the lesions of reversal reactions or ENL which these patients eventually developed, there was a significant thickening of the nucleated epidermis in both reactional states. In both comparison groups, there was a greater mean increase and a larger frequency of thickening in the ENL lesions than in those with reversal reactions. In the borderline tuberculoid and reversal reaction lesions the increase can be understood as secondary to the presence of gamma interferon or interleukin-2. The increase in thickness in the ENL lesions is more difficult to explain, but it is not inconsistent with a role for these same two cytokines.RÉSUMÉ
A partir de 28 échantillons de peau cliniquement normale provenant de sujets souffrant de lèpre lépro-mateuse comme référence de comparaison, l'épaisseur moyenne de l'épiderme avec noyaux cellulaires (nucléé) fut déterminée être significativement augmentée parmi les lésions non-traitées de 16 patients tuberculides borderlines, 21 patients souffrant d'érythème noueux lépreux (ENL) et 14 patients présentant des réaction inverses, alors qu'elle restait inchangée parmi les lésions non traitées des patients lépromateux borderlines et les patients lépromateux. En utilisant les prélèvements de 36 lésions lépromateuses et lépromateuses borderlines non-traitées comme référence de comparaison avec celles des réactions inverses ou d'ENL que ces patients ont ensuite développés, il fut trouvé un épaississement significatif de l'épiderme nucléé dans les deux états réactionnels. Au sein des 2 groupes de comparaisons, l'augmentation moyenne d'épaisseur de l'épiderme nucléé et la fréquence de l'épaississement furent plus importante parmi les lésions d'ENL que parmi les lésions de réactions inverses. Cette augmentation d'épaisseur de l'épiderme peut être interprétée comme urge réponse à la présence d'interféron gamma et d'interleukine 2 parmi les lésions tuberculoïdes borderlines et celles des réactions inverses. L'augmentation d'épaisseur au sein des lésions d'ENL est plus difficile à expliquer, mais il n'est pas improbable que ces 2 mêmes cytokines jouent un rôle au sein des lésions d'ENL.RESUMEN
Usando 28 especimenes de piel clinicamente normal de pacientes con lepra lepromatosa como estándar de comparación, se encontró que el grosor promedio de la epidermis nucleada estuvo significativamente aumentado en las lesiones no tratadas de 16 pacientes con lepra tuberculoide subpolar (BT), 21 pacientes con eritema nodoso leproso (ENL) y 14 pacientes con reacciones reversas, pero se mantuvo sin cambio en los pacientes con lepra lepromatosa subpolar (BL) y lepra lepromatosa (LL). Por otro lado, comparando 36 especimenes de lesiones de lepra lepromatosa y lepra lepromatosa subpolar con las lesiones de reacciones reversas o ENL que estos pacientes desarrollaron, se observó un engrosamiento significativo de la epidermis nucleada en ambos estados reaccionales. En los dos grupos comparados hubo un mayor incremento promedio y una mayor frecuencia de engrosamiento en las lesiones ENL que en las de reacción reversa. En las lesiones BT y de reacción reversa el incremento puede entenderse como secundario a la presencia de interferón gamma o de interleucina-2. El aumento en el grosor en las lesiones ENL es más dificil de explicar, pero también podría estar relacionado con estas mismas citocinas.In his review of the histology of cutaneous delayed-type hypersensitivity (DTH) reactions, Turk cited epidermal thickening as a common change in tuberculin responses and allergic contact dermatitis, both in people and in guinea pigs (14). Studying positive tuberculin responses in patients with leprosy, Kaplan, et al. (4) found an increase in epidermal thickness, as much as twofold or more, comparing tuberculin injected lepromatous leprosy (LL) lesions to uninjected, control lesions. Also reported in that study was epidermal thickening in many of the tuberculoid, i.e., polar tuberculoid (TT) or borderline tuberculoid (BT), lesions. The intradermal administration of gamma interferon (IFN-γ) (7) or interleukin-2 (IL-2)(3), cytokines important in the pathogenesis of DTH reactions, into treated LL patients also resulted in epidermal thickening, thus providing a possible mechanistic framework for understanding the observed epidermal change. Two groups (11, 13) have reported epidermal thickening to be a common change in erythema nodosum leprosum (ENL) and one has reported this change in reversal reactions (RRs) as well (13).
To establish a more detailed perspective on the extent and frequency of thickening of the nucleated epidermis (NED) throughout the clinical manifestations of leprosy, a retrospective analysis of histologic material was undertaken and is reported herein.
MATERIALS AND METHODS
Patients in the Hansen's Disease Clinic of the Los Angeles County/University of Southern California Medical Center, Los Angeles, California, U.S.A., were classified according to the criteria and nomenclature of Ridley and his colleagues (10). Criteria for the diagnosis of ENL and RRs were as previously described (9). Although RRs and DTH reactions may be synonyms in the context of leprosy, in this paper, to avoid ambiguity, DTH will be used only in its generic sense and not as a name for RRs.
The specimens of clinically normal skin included in this study were some of those previously reported (8): two from borderline lepromatous (BL) subjects, the rest LL. The remainder of the material studied was obtained for routine diagnostic purposes. All specimens were processed routinely for paraffin embedding and staining with hematoxylin and eosin (H&E).
The thickness of each NED was calculated by a photomicrographic method, thus yielding an average thickness for a structure of variable thickness. The low-power objective (4×) was used to place the middle of the epidermis in the center field and to orient the long axis of the epidermis parallel to the long axis of the photomicrographic field. The 10× objective was then placed over the center of the epidermis without disturbing its orientation in the photographic field. A photograph was obtained with the 10× objective using Kodak Gold film for color prints, ASA 200. Commercially processed 6 × 4 inch color prints were obtained from each photograph. In all photographs the epidermis extended to the two shorter edges of the photograph. The weight of each print and of a cutout of the NED was determined, and the area of the NED was expressed as a percent of the weight of the entire photograph. In order to express the thickness of the NED as an average linear measurement, a hemocytometer with markings 330 microns wide, confirmed by calibrated reticule, was similarly photographed and weighed, repeated weights being 37.7% of the total weight of the sheet. Thus, 1 micron in thickness was equivalent to 0.1142% of the weight of the sheet, when using the 10× objective. The average thickness of each NED in microns was obtained by dividing its area in percent by 0.1142.
In one untreated LL patient who presented with ENL and diffuse non-nodular LL, a biopsy of an ENL lesion was obtained from one arm and another from clinically normal skin from a symmetrical site on the opposite side. Photomicrographs of these specimens are included for illustration purposes only (Fig. 3A and B).
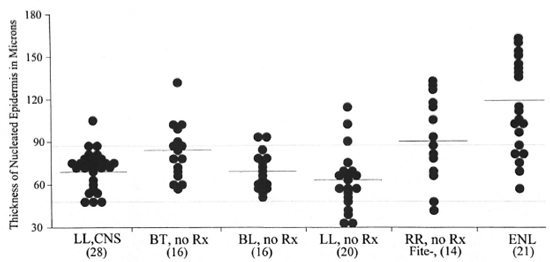
Fig. 1. A plot of the thickness of the nucleated epidermis for the various groups studied, excluding those paired to a reactional state. Mean values are indicated by the solid lines. Dotted lines indicate the 87 and 47 micron values. CNS = Clinically normal skin; Rx = treatment; RR = reversal reaction; numbers in parentheses; Fite- = negative for acid-fast bacilli on Fite stains.
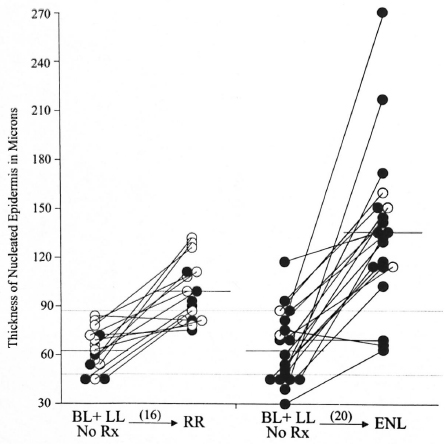
Fig. 2. A plot of the thickness of the nucleated epidermis in the lesions paired with an eventual reactional state. The solid circles represent LL patients, the open circles represent BL patients. The line connecting the circles indicates lesions from an individual patient. Mean values are indicated by a solid line. The dotted lines indicate the 87 and 47 micron values.
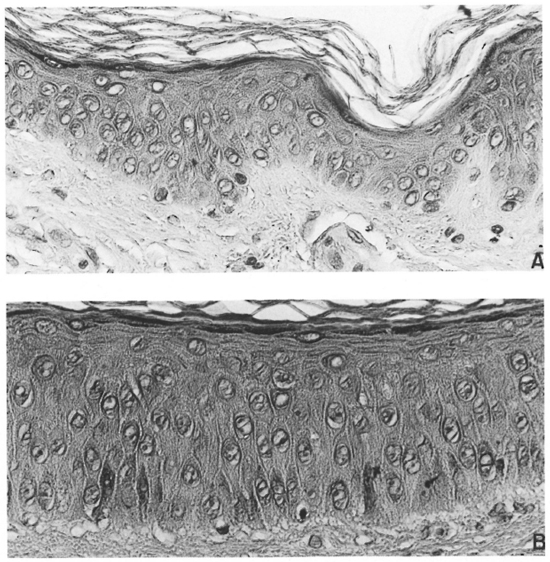
Fig. 3. A = Epidermis from clinically normal skin of a patient with diffuse, non-nodular lepromatous leprosy and B = Epidermis from a lesion of ENL from a symmetrical site (×40). The epidermis of the ENL lesion is clearly thicker, has a better developed columnar habitus to the basal cells, a columnar habitus to at least two layers above the basal layer, and little flattening of the keratinoeytes as the granular cell layer is approached.
Figures 1 and 2 were made on a personal computer using the Microsoft Power Point 97 program. Each data point is a circle 3 pixels in diameter. The scale is such that 30 microns are equivalent to 10 pixels, and each data point was necessarily rounded off to the nearest pixel, such as that representing 60, 63, 69, 72 microns, etc. This approximation was considered to be well within the error of the method. Statistical analysis utilized the Microsoft Excel program, assuming a normal distribution and unequal variances. A p value of <0.05 was considered to be significant.
RESULTS
The thickness of the NED was measured in 187 specimens obtained from 151 patients. Figure 1 summarizes the data from clinically normal skin and from all patients except those paired with a reactional state.
The mean value of thickness of the NED from clinically normal skin from 28 specimens, 26 from an arm and 2 from the back, was 70 with a range of 48-104 microns. As can be seen in Figure 1, in 27 specimens the NED was in a narrow range, 48-87 microns, indicating that any value 90 microns or more should be regarded as "thickened" at least for frequency comparison. For the NED to be considered "thinned," the value of 47 microns or less was taken.
The mean value for thickness of the NED from 16 untreated BT patients, 85 microns, differed significantly from that of clinically normal skin (two-tail p <0.02) and 5 of the 16 specimens (31%) were 90 microns or greater in thickness. In the 16 untreated BL lesions, the mean thickness, 69 microns, did not differ significantly from that of clinically normal skin, but 2 (13%) were 90 microns or greater. Also, in the 20 untreated LL lesions the mean thickness, 62 microns, was not significantly less than that of clinically normal skin, but 3 specimens (12%) were 90 microns or greater.
In the 14 specimens of untreated, negative for acid-fast bacilli (AFB) on Fite stain RRs, the mean thickness, 90 microns, was significantly greater than that of clinically normal skin (two tail p <0.04) and seven (50%) had values in excess of 90 microns. In the 21 patients with untreated ENL the mean thickness, 118 microns, was also significantly greater than that of clinically normal skin (two-tail p <0.0001) and 15 (71%) had values in excess of 90 microns. The mean NED thickness of the ENL lesions was significantly greater than that of the RR lesions (two-tail p <0.03).
Figure 2 summarizes the data obtained from two groups of untreated LL and BL patients, one group of whom eventually developed ENL; the other group, RRs. Among the two groups of LL and BL patients whose untreated biopsy specimens were paired with those of their eventual reaction, the mean thickness values of untreated lesions in both groups, 62 microns and 63 microns, respectively, were similar and did not differ significantly from those of clinically normal skin or the separate, unpaired LL and BL groups. Among the 16 individuals who eventually developed RRs, none had a baseline specimen in excess of 87 microns. Among the 20 individuals who eventually developed ENL, two (10%) of baseline specimens had a NED thickness of 90 microns or greater. When these individuals went into reaction, those who developed RRs had a mean lesional NED thickness of 99 microns, significantly greater than the baseline value (two-tail p <0.01) and nine (56%) had values of 90 microns or greater. Also 13 of the 16 (81%) had a thickening of the epidermis, even if not 90 microns or greater. The mean ratio of the increase in those developing RRs (reactional/nonreactional) was 1.72.
Of those pairs who developed ENL, the mean lesional NED thickness was 134 microns, significantly greater than the baseline value (p <0.0001) and 17 (85%) had values in excess of 90 microns. Also 18 of the 20 (90%) had a thickening of the epidermis. The mean ratio of increase in those developing ENL was 2.27, significantly greater than that for those developing RRs (two-tail p <0.04). One entirely untreated patient in this group had two biopsies on the same occasion, one of a lepromatous nodule and the other from an ENL lesion from a symmetrical site on the arms, the NED of the former measuring 73 microns and the latter 271 microns.
Fifteen specimens had a NED considered to be "thinned," i.e., less than 47 microns. This occurred primarily in untreated lepromatous patients, 13 or 87%. A "thinned" NED was unusual among BL or RR patients, 1 in each or 7%. If 46 microns is taken to be the lower limit of normal, then only 7 specimens would be "thinned," 6 LL and 1 RR, the latter being the only facial specimen in this series.
Figures 3A and 3B demonstrate the differences between an ENL lesion and the clinically normal skin from a symmetrical site, the patient presenting with untreated diffuse non-nodular LL and ENL. The differences in thickness and the number of layers of nucleated keratinocytes are clear. The normal columnar habitus of the basal cell layer is scarcely discernable in the clinically normal skin but is well developed in the ENL lesion. In addition, a columnar shape is also well developed in the first two prickle cell layers, and in the upper portion of the prickle cell layer of the ENL lesion the normal "flattening" of the keratinocytes is attenuated.
DISCUSSION
The presence of statistically significant NED thickening in BT, RR, and ENL lesions is in accord with the prior observations of others (4, 11, 13). That 50% of Fite stain-negative and 75% of Fite stain-positive RR lesions have a thickened NED is putatively explained by the large amounts of mRNA coding for IFN-γ and IL-2 found in the dermis of such lesions. Either agent when administered intradermally resulted in NED thickening (3, 7). This is also a reasonable explanation for the thickening found in BT lesions. A good explanation for the more frequent and greater thickening found in ENL lesions is more difficult, the mRNA coding for IFN-γ and IL-2 not being so abundant (15). However, other techniques have demonstrated increased levels of both IFN-γ (1) and IL-2 (5) in ENL lesions, thus making it difficult to exclude these agents as having no role in the development of the thickened NED. Also, the precipitation of ENL by the administration of INF-γ is consistent with a role for this cytokine in the pathogenesis of ENL (12).
As can be seen in Figures 1 and 2, specimens with a thick NED were found in all but one nonreactional BL and LL group examined. These apparent outliers might be dismissed as meaningless random events, variations in the anatomical site of the lesions, or as errors of tissue processing, such as oblique sectioning of the tissues. Because no evidence of oblique sectioning was found, such as a wide granular cell layer or a net-like appearance to the epidermal retia, and because chart reviews of the 72 paired biopsy specimens whether nonreactional or reactional showed no apparent variation in NED thickness from the three sites sampled most frequently (arm 39%, thigh 18%, back 14% or not stated 17%), biologically important explanations warrant consideration, even though purely random events cannot be excluded. For the nonreactional lesions which show NED thickening a "pre-reactional" condition could be postulated, but it is only a matter of speculation at present.
In two different populations, the NED in ENL was significantly thicker than that in RR. This is consistent with each having separate pathogenic mechanisms and, therefore, perhaps different mediators. Alternatively, ENL lesions are brief and, hence, uncompensated acute changes predominate; whereas in the longer-lived RR lesion there is opportunity for a new equilibrium to be established, producing the comparatively thinner NED. Consistent with the latter is a lesser mean NED thickness value, 90 microns, for the specimens obtained from patients who had RRs as their presenting manifestation of leprosy, the Fite stain-negative de novo group as shown in Figure 1, patients whose lesions were of relatively long duration (median time to biopsy 10 weeks, range 4 to 26 weeks); whereas a greater mean NED thickness, 99 microns, was present in the specimens from patients who were already under care when the RRs developed, the Fite stain-positive group as shown in Figure 2, patients who had easy and prompt access to the clinic and whose lesions were of shorter duration (median time to biopsy 2.5 weeks, range 4 days to 4 weeks).
The anatomical changes demonstrated in Figures 3A and 3B are exemplary of the observed changes found in most but not in all "thickened" specimens. However, compared with nonreactional BLor LL tissues, a better developed columnar appearance to basal cells was seen in a few BT, RR, and ENL specimens which had no "thickened" NED (data not shown), suggesting that this change alone perhaps should arouse suspicion that one of these three conditions might be present.
Two kinds of errors are inherent in the method used, but neither were considered to be confounding of the observed "significant" differences. One error is a consequence of variation in the weight of individual photographic prints and, its corollary, that variation must also be present within any given print. Because this error would be random, that is to say, would overestimate as often as underestimate the thickness of the NED, such errors would cancel out each other. The other error is a consequence of a commonly occurring curvature to the epidermis, the outer surface being a convexity. The curvature occurs in a majority of specimens, thus giving rise to a systematic error which overestimates the NED thickness. However, because the error appeared to be present in similar frequency and extent in all groups studied, it was considered not to invalidate the statistical results. Also, in a preliminary study using 20× and 40×, as well as 10× , objectives, where the curvature-induced error was less with the larger objectives, the distribution of values for NED thickness was wider with the larger objectives, a finding indicating that the 10× objective obtained a "better" or more representative sample of all of the variations in epidermal thickness. This curvature error could explain in part the larger mean value for NED thickness (62 microns) in LL lesions in the present study as contrasted to the smaller value (48 microns) reported by Kaplan, et al. (3).
The nomenclature concerning the epidermis may lead to confusion. Unqualified "epidermis" refers to both the nucleated cellular layers and non-nucleated cells of the stratum corneum. In the referenced papers (4, 7, 11, 13, 14), it is implied that what is thickened is the NED, because the authors also mention an increase in cellular layers which are not countable in the stratum corneum of formalin-fixed material. The term "malpighian layer" is used by some (6) to be identical with the NED, but by others (2) to mean either the basal cell layer and the prickle cell layer or only the basal cell layer. Hence, the clarity of the term "NED" compensates for its awkwardness.
REFERENCES
1. Cooper, C. L., Mueller, C., Sinchaisri, T.-A., Pirmez, C., Chan, J., Kaplan, G., Young, S. M. M., Weissman, I. L., Bloom, B. R., Rea, T. H. and Modlin, R. L. Analysis of naturally occurring delayed-type hypersensitivity reactions in leprosy by in situ hybridization. J. Exp. Med. 196 (1989) 1565-1581.
2. Dorland's Illustrated Medical Dictionary. 28th edn. Anderson, D. M., Chief Lexicographer. Philadelphia: W. B. Saunders, 1994.
3. Kaplan, G., Kiessling, R., Teklemariam, S., Hancock, G., Sheftel, G., Job, C. K., Converse, P., Ottenhoff, T. H. M., Becx-Bleumink, M., Deitz, M. and Cohn, Z. A. The reconstitution of cell-mediated immunity in the cutaneous lesions of lepromatous leprosy by recombinant interleukin-2. J. Exp. Med. 169 (1989) 893-907.
4. Kaplan, G., Witmer, M. D., Nath, I., Steinman, R. M., Laal, S., Prasad, H. K., Sarno, E. N., Elvers, U. and Cohn, Z. A. Influence of delayed immune reactions on human epidermal keratinocytes. Proc. Natl. Acad. Sci. U.S.A. 83 (1986) 3469-3473.
5. Modlin, R. L., Hofman, F. M., Horwitz, D. A., Husmann, L. A., Gillis, S., Taylor, C. R. and Rea, T. H. In situ identification of cells in human leprosy granulomas with monoclonal antibodies to interleukin-2 and its receptor. J. Immunol. 132 (1984) 3085-3090.
6. Murphy, G. F. Histology of the skin. In: Lever's Histopathology of the Skin. 8th edn. Elder. D., ed. Philadelphia and New York: Lippincott-Raven, 1997, p. 6.
7. Nathan, C. F., Kaplan, G., Levis, W. R., Nusrat, A., Witmer, M. G., Sherwin, S. A., Job, C. K., Horowitz, C. R., Steinman, R. M. and Cohn, Z. A. Local and systemic effects of intradermal recombinant interferon-γ in patients with lepromatous leprosy. N. Engl. J. Med. 315 (1986) 6-15.
8. Rea, T. H., Gottlieb, B. and Levan, N. E. Apparently normal skin in lepromatous leprosy. Arch. Dermatol. 111 (1975) 1571-1574.
9. Rea, T. H. and Sieling, P. A. Delayed-type hypersensitivity reactions followed by erythema nodosum leprosum. Int. J. Lepr. 66 (1998) 316-327.
10. Ridley, D. S. Histological classification and the immunological spectrum of leprosy. Bull. WHO 51 (1977) 451-465.
11. Sampaio, E. P., Kaplan, G., Miranda, A., Nery, J. A. C., Miguel, C. P.. Viana, S. M. and Sarno, N. S. The influence of thalidomide on the clinical and immunologic manifestation of erythema nodosum leprosum. J. Infect. Dis. 168 (1993) 408-414.
12. Sampaio, E. P., Moreira. A. L., Sarno, E. N., Malta, A. M. and Kaplan, G. Prolonged treatment with recombinant interferon-γ induces erythema nodosum leprosum in lepromatous leprosy patients. J. Exp. Med. 175 (1992) 1729-1737.
13. Thangaraj, H., Laal, S., Thangaraj, I. and Nath, I. Epidermal changes in reactional leprosy: keratinocyte la expression as an indicator of cellmediated immune responses. Int. J. Lepr. 56 (1988) 401-407.
14. Turk, J. L. Delayed Hypersensitivity. Amsterdam: Elsevier North-Holland, 1975.
15. Yamamura. M., Wang, X.-H., Ohmen, J. D., Uyemura, K., Rea, T. H., Bloom, B. R. and Modlin, R. L. Cytokine patterns of immunologically mediated tissue damage. J. Immunol. 149 (1992) 1470-1475.
M.D.. Division of Dermatology, Keck School of Medicine, University of Southern California, Los Angeles, California, U.S.A.
Reprint requests to T. H. Rea, M.D., Division of Dermatology, LAC/USC Medical Center, 1200 N. State Street Room 8440, Los Angeles. CA 90033, U.S.A. or fax 1-323-226-2654; email: rea@hsc.usc.edu
Received lor publication on 18 July 2000.
Accepted for publication on 7 December 2000.