- Volume 66 , Number 1
- Page: 53
Survival of HIV-Positive and HIV-Negative leprosy patients in Mwanza, Tanzania
This department is for the publication of informal communications that are of interest because they are informative and stimulating, and for the discussion of controversial matters. The mandate of this JOURNAL is to disseminate information relating to leprosy in particular and also other mycobacterial diseases. Dissident comment or interpretation on published research is of course valid, but personality attacks on individuals would seem unnecessary. Political comments, valid or not, also are unwelcome. They might result in interference with the distribution of the JOURNAL and thus interfere with its prime purpose.
To the Editor:
Little is known on the long-term survival, relapse and incidence of complications among leprosy patients with and without the human immunodeficiency virus (HIV) infection. There are case reports suggesting an association of HIV infection with reversal reaction (RR) and neuritis as well as with increased incidence and mortality due to severe erythema nodosum leprosum (ENL) reactions (2,4,6). There are no reports suggesting that HIV infection predisposes a leprosy patient to relapse following a curative course of treatment. It is important to know whether a difference exists in the outcome of treatment and the risk of complications after treatment in relation to HIV infection.
Between 1 April and 30 September 1991, 120 consecutively diagnosed new and relapsed after multiple drug therapy (MDT), self-reported, multibacillary (MB) and paucibacillary (PB) leprosy patients in the Mwanza region in Tanzania were enrolled in a study of the association of leprosy with HIV infection. The diagnosis, classification, outcome of treatment and survival was assessed under routine program conditions by experienced District Tuberculosis and Leprosy Coordinators (DTLCs). Classification was still based on skin-smear results in addition to the clinical picture. Detailed descriptions of the study methods have been described earlier (1,3). PB patients were treated with six monthly supervised doses of rifampin and daily self-administered Isoprodian (combination of dapsone, prothionamide and isonia/.id) and were assessed after 1 year. MB patients were treated with 24 monthly supervised doses of rifampin and daily self-administered Isoprodian and were assessed after 3 years. The outcome of "Treatment completed" was considered when the patient had finished treatment as prescribed. In case of HIV infection, no therapy for HIV was given.
During October and November 1995 a follow-up assessment among the same patients was performed. DTLCs made all possible efforts to interview the patients at their homes or to question their relatives and neighbors and trace patients who had moved in order to establish their current status. Relapses were diagnosed according to the National Tuberculosis and Leprosy Programme protocols.
For patients who had died after completing treatment, the cause of death was determined by verbal autopsy, following the protocol of the adult morbidity and mortality study performed elsewhere in Tanzania (McLarty, D. Policy implications of adult morbidity and mortality; preliminary report. Dar es Salaam: Dar es Salaam University Medical Faculty, 1993).
Out of 120 patients enrolled, 42 (35%) had MB leprosy. This proportion has been similar over the years in the Mwanza region. Among these 42 MB patients HIV infection was higher than in the PB patients, although statistically not significant. An association between HIV infection and MB leprosy was reported earlier in a population-based, case-control study of a subset of these patients (those between 15 and 54 years of age), suggesting that HIV is a risk factor for MB leprosy (1). The overall HIV seropositivity among the leprosy patients was 7.5% (9/120). The highest HIV seroprevalence was found in the 25 to 34 age group. None of the six cases diagnosed as a relapse of leprosy was HIV-positive (Table 1).
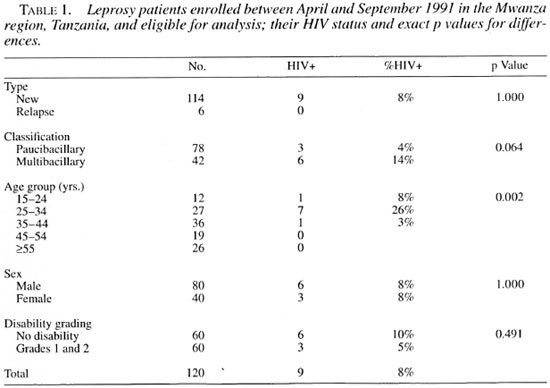
The treatment completion rate for HIV-positive patients was 44% (4/9); for HIV-negative leprosy patients, 85% (94/111). This was due mainly to the differences in mortality in HIV-positives (3/9) and HIV-negatives (2/111), and was statistically significant (odds ratio 5.1, 95% confidence interval 1.1-24.1) also after adjusting for age, sex and disability grading. Adjustment for classification has been omitted because of collinearity between MB leprosy and HIV seropositivity and subsequent mortality.
At the end of treatment five patients had died. At the time of follow up after 55 months, an additional five patients had died, all of whom were HIV-negative at the moment of enrollment into the study (Table 2). Only four patients could not be located for reassessment. Among the 106 patients who could be assessed after 55 months, there was no evidence of relapse or having developed a leprosy reaction after finishing treatment.
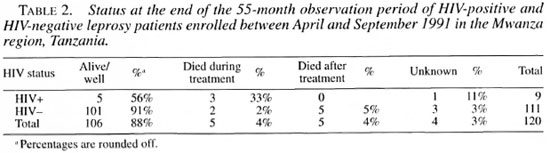
The overall mortality of the HIV-positive patients (3/9) was statistically significantly greater than that of HIV-negative patients (7/111). Of the 10 patients who died, 9 were MB and 1 was PB. Verbal autopsies were done for all five patients who had died after treatment (all HIV-negative at time of diagnosis) but not for the five patients who had died during treatment. The reported causes of death were not related to HIV infection or to AIDS: heart failure in two patients, snake bite in the third, and unknown in the fourth patient. In the fifth, a severe case of ENL was the underlying cause of death.
Survival analysis according to the Cox proportional hazard model showed that the probability of dying was significantly associated with HIV infection but not with sex, age group or disability grading. HIV-positive patients had an 8.3 higher chance of dying than did HIV-negative patients.
The higher early mortality after diagnosis could be explained by a more advanced stage of HIV infection in MB leprosy patients at the moment of the diagnosis. This is further supported by a similar survival curve among HIV-positive tuberculosis patients (8) enrolled in the same period in the Mwanza region (The Figure).
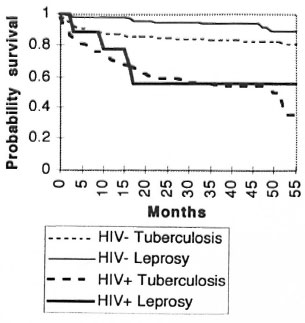
The Figure. Kaplan Meier survival curve for HIV-positive and HIV-negative tuberculosis and leprosy patients enrolled between April and September 1991 in the Mwanza region, Tanzania.
Although not confirmed in this study, in Tanzania a higher proportion of MB patients have disability and have a higher mean age at the moment of diagnosis than do PB patients. This suggests that MB patients tend to wait longer before coming forward for diagnosis and treatment (van den Broek, J., Lubango, M., van der Steen, F., Rubona, J. and van Wijnen, A. Prevention of disabilities and rehabilitation of leprosy patients in Tanzania: estimating the prevalence of persons affected by leprosy; unpublished data and Annual Reports 1984 through 1995 of the National Tuberculosis and Leprosy Programme, Tanzania). The long latent period of MB leprosy, in combination with the longer patient delay, would expose these patients longer to the risk of contracting HIV infection before being diagnosed as a leprosy patient. It is also unknown to what extent undiagnosed HIVpositive MB patients may have died before the diagnosis of leprosy was made. We also could not rule out a possible effect of falsepositive results of the ELISA in relation to leprosy, as is reported to exist (5).
The assumption that HIV infection, in combination with MB leprosy, may lead to more frequent and serious complications of leprosy, such as ENL and RR, could not be confirmed in this study. No relapse or reaction at the time of examination or during the observation period following treatment could be shown to have occurred, contrary to the findings in a group of 18 HIV-positive leprosy patients and 18 HIV-negative matched controls in Kenya (7). This finding casts some doubt on the accuracy of the recall of the informants, or the ascertainment of the physical condition of the patients by the interviewers.
The mortality rate among leprosy patients in the 1984 to 1995 annual treatment cohorts of the national data in Tanzania did not show any change. It also has been consistently higher among MB (on the average 3%) than among PB patients (on the average 0.8%). Any effect of the HIV pandemic on a change in the mortality rate during treatment of leprosy could, therefore, not be confirmed by the national data. The number of patients and deaths in this study was small, and therefore the results should be interpreted with caution.
- Jacques van den Broek, M.D., M.P.H.
National Tuberculosis and Leprosy Programme
P.O. Box 5478
Dar es Salaam, Tanzania
Royal Tropical Institute
Wibautstraat 137J
1097 DN Amsterdam, The Netherlands
- Sayoki Mfinanga, M.D.
National Institute of Medical Research
Muhimbili Research Station and Central Tuberculosis Laboratory
P.O. Box 3436
Dar es Salaam, Tanzania
- Candida Moshiro, M.Sc.
Department of Epidemiology and Biostatistics
University of Dar es Salaam
P.O. Box 65001
Dar es Salaam, Tanzania
- Richard O'Brien, M.D.
Tuberculosis Unit
World Health Organization
Geneva, Switzerland
Division ofTB Elimination
Centers for Disease Control and Prevention
Atlanta. Georgia 30333, U.S.A.
- Arbogast Mugomela
Laboratory Technician
Bugando Medical Centre
P.O. Box 1370
Mwanza, Tanzania
REFERENCES
1. Borgdorff, M., Van Den Broek, J., Chim, H., Klokke, H., Graf, P., Barongo, L. R. and Newell, J. N. HIV-1 infection as a risk factor for leprosy; a case-control study in Tanzania. Int. J. Lepr. 61(1993)556-562.
2. Bwire, R. and Kawuma, H. J. Type I reactions in leprosy, neuritis and steroid therapy; the impact of the human immunodeficiency virus. Trans. R. Soc. Trop. Med. Hyg. 88(1994)315-316.
3. Chum, H. J., O'Brien, R. J., Chonde, T. M., Graf, P. and Rieder, H. L. An epidemiological study of tuberculosis and HIV infection in Tanzania. 1991-1993. AIDS 10(1996)299-309.
4. De Almeida, A. M., Ferreira Roselino, A. M. and Tiraboschi Foss, N. Leprosy and HIV infection. (Letter) Int. J. Lepr.62(1994)133-135.
5. Kashala, O., Marlink, R., Ilunga, M., Diese, M., Gormus, B., Xu, K., Mukeba, P., Kasongo, K. and Essex, M. Infection with human immunodeficiency virus type 1 (HIV-1) and human T cell lymphotropic viruses among leprosy patients and contacts: correlation between HIV-1 cross reactivity and antibodies to lipoarabinomannan. J. Infect. Dis. 169(1994)296-304.
6. Olivares, L. M., Pizzariello, G. E. A., Benetucci, J. and Farina, M. H. Lepromatous leprosy and HIV infection. (Letter) Int. J. Lepr.62(1994)295-296.
7. Orege, P. A., Odawa, B. A., Okello, C. M., Obura, M. Okuku, P., Amimo, R. K. and Were, M. The effect of HIV infection on clinical response of leprosy patients to multiple drug therapy in Kenya. (Abstract) Int. J. Lepr.61 (1993) 37A.
8. van Den Broek, J., Mfinaga, S., Moshiro, C, O'Brien, R., Mugomela, A. and Lefi, M. Impact of HIV infection on the outcome of treatment and survival of tuberculosis patients in Mwanza. Tanzania. Int. J. Tuberc. Lung Dis. (accepted for publication 1997).
Reprint requests to J. van den Broek, Van Ommerenstraat 16, 5708 KB Helmond, The Netherlands.