- Volume 66 , Number 1
- Page: 10–5
Leprosy reactions - complications of steroid therapy
ABSTRACT
The adverse effects of corticosteroid therapy while treating 830 patients suffering f rom leprosy reaction (type 1 =581 ; type 2 = 249) are presented. Some of the adverse effects were cosmetically distressing, while others were disabling. Patients suffering f rom type 2 reaction - because of the tendency of the reaction to recur over a long time - needed steroids for a longer duration; hence, adverse effects were more frequent. Measures to counter some of the adverse effects are suggested and the need to identify drugs with potentially less adverse effects is emphasized.RÉSUMÉ
Les effets secondaires du traitement par corticostéroides de 830 malades souffrant d'une réaction lépreuse (type 1 =581; type 2 = 249) sont présentés. Certains effets secondaires étaient de nature cosmétique, d'autres de type incapacitant. Les patients soufrrant d'une réaction de type 2, á cause d'une tendance éla récidive sur une longue période, nécessitaient un traitement par stéroides plus long; il s'ensuivait des effets secondaires plus fréquents. Des mesures pour contrer certains effets secondaires sont suggérées et la nécessité d'identifier des médicaments avec potentiellement moins d'effets secondaires est soulignée.RESUMEN
Se analizaron los efectos adversos de los corticoesleroides en el tratamiento de la reacción lipo 2) recibieron la terapia esteroidal. Algunos de los electos adversos fueron sólo de tipo estético mientras que otros fueron descapacitantes. Los pacientes con reacción leprosa tipo 2, debido al comportamiento recurrente de la reacción, necesitaron un tratamiento esteroidal más prolongado y por esta razón los efectos adversos fueron más frecuentes. Se sugieren algunas medidas para contrarrestar algunos de los efectos adversos y se enfatiza la necesidad de buscar drogas con menos efectos indeseables.Leprosy is a chronic granulomatous disease caused by Mycobacterium leprae. Some patients develop acute inflammatory episodes due to an adverse immune response to bacterial antigens, and these episodes are called leprosy reactions. Many classifications have been proposed for these reactions, but the classification proposed by Jopling is commonly followed in many places and is used here.
Reactions
Type 1 reaction. Type 1 reaction occurs in borderline tuberculoid (BT), borderline borderline (BB) and borderline lepromatous (BL) patients (synonyms: reversal reaction, upgrading reaction, borderline reaction). It occurs as a cell-mediated, delayed-type hypersensitivity (DTH) response.
Type 2 reaction. Type 2 reaction occurs in BL and lepromatous (LL) patients [synonym: erythema nodosum leprosum (ENL)]. It occurs as an immune-complex mediated response. This reaction can last from 3 months to 6 years.
The detailed clinical descriptions of these reactions, their immunopathological sequences and mplications are beyond the scope of this article, but excellent publications are available 4,17,28,30).
Leprosy reactions can occur in patients before treatment, during treatment, and even after treatment (3,7,19,20,30). Reactions frequently cause nerve damage which leads to disability and deformity. Corticosteroids are frequently used to control reactions(2,13,19,20,30). Corticosteroids are effective in the management of reactions in the following ways (20): They suppress immune responses (both DTH and immune complex), reduce inflammation, and prevent fibrosis.
Although caution about the adverse effects of steroid therapy has been given by many, to our knowledge the exact incidence of adverse effects relating to steroid therapy has not been described in detail in the literature (20). We present here our observations of adverse effects of steroid therapy.
PATIENTS AND METHODS
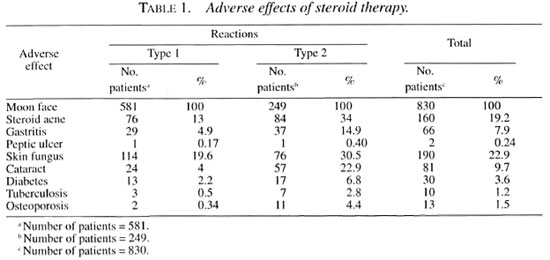
Patients with reactions who came to the author between January 1991 and December 1996 were included in the study, and were suffering from the following reactions: type 1, 581; type 2, 249; total 830.
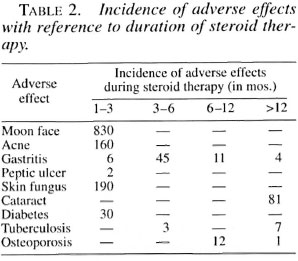
Prednisolone was the usual corticosteroid used for therapy along with an appropriate regimen of multidrug therapy (MDT). Adverse effects were carefully looked for and recorded. The usual starting dose of prednisolone was 40-60 mg/day, given as a single dose in the morning. If symptoms such as fever, neuritis and arthritis did not subside within 2 days the daily dosage was increased. Occasionally patients needed up to 120 mg of prednisolone/day for 2 or 3 days after which the dosage could be reduced. The adverse effects are shown in Tables 1 and 2.
Adverse effects
Moon face. Cushingoid features are common in patients under chronic corticosteroid therapy and "moon face" was the commonest feature seen in all of our patients after receiving corticosteroids even for 2 weeks (11). Patients and their relatives felt very anxious and even suspected "kidney problems." We had to reassure the patients about the temporary nature of this phenomenon and that everything would subside after the steroid course was over.
Steroid acne. Steroid acne was a cosmetically disturbing problem which occurred quite commonly after about 1 month of steroid therapy. The common sites of distribution of these eruptions were the face, chest, arms and scapula. This, like "moon face," also was temporary and disappeared after steroid therapy was completed.
Gastritis. Patients complaining of a burning sensation in the stomach or epigastric distress were considered as suffering from gastritis. This usually occurred any time during steroid therapy (less than 6 months of therapy or beyond 6 months of therapy) and with any dosage. Occasionally there was also vomiting. All of these patients were managed with oral antacids and ranitidine without any interruption of the steroid therapy. When the vomiting was severe, we administered ranitidine and steroids parenterally along with intravenous fluids for a few days until the gastritis subsided. We also suspect that clofazimine might have contributed to these symptoms in some patients on multibacillary MDT (14).
Peptic ulcer. Following are two examples of peptic ulcer: 1) A 35-year-old woman with type 1 reaction developed severe abdominal pain 2 weeks after taking prednisolone 30 mg daily. She was found to have a perforated peptic ulcer at laparotomy. The steroid therapy was discontinued in this patient. 2) a 40-year-old man with severe type 2 reaction and a history of alcoholism developed profuse hematemesis 1 week after prednisolone therapy (30 mg/day). The patient died of shock before a blood transfusion could be arranged. Even though this is a serious complication, its incidence is quite rare, contrary to popular belief (8,11).
It is possible that we might have missed some of the asymptomatic silent ulcers since we relied only on clinical symptoms.
Fungus infection of the skin. Skin fungus occurred quite commonly, probably due to immune suppression during steroid therapy. Itchy plaques occurred over the thighs, abdomen, chest and axillae, groin and gluteus. This usually was controlled with topical antifungal agents (clotrimazole or miconazole) and occasionally a patient needed oral griseofulvin as well.
Cataract. The annoying problem of cataract occurred quite frequently in patients who were on steroids for more than 12 months (16,11,31). Generally, in the initial stage patients suffered from "glare disability" disturbing their vision when they were outdoors under bright sunlight (37). The visual difficulty was described as "foggy," "cloudy" and "smoky," but vision dramatically improved after dusk. Vision also improved in clarity if they wore dark sunglasses during bright sunlight. Because of this glare disability, initially there was difficulty in recognizing distant objects and in some patients, as the cataract formation intensified, there was difficulty in recognizing near objects, greatly compromising their day-to-day activities (difficulty in recognizing friends and foe, difficulty in reading the number on a bus when boarding, difficulty during manual weed removal and harvest work and, of course, in reading fine print in the newspaper). Those driving at night felt "blinded" by the headlights of oncoming vehicles. The experience was described as a "smoke screen" or "colorful dazzling screen," akin to driving on a rainy night without the windshield wipers working.
In the initial stages even though the patients suffered from glare disability and difficulty in recognizing distant objects, visual acuity in Snellen's chart was found to be normal or near normal (6/6 or 6/9). Hence, testing for the glare disability and contrast sensitivity using Pelli-Robson's chart may help to detect the magnitude of this problem much earlier (37).
The incidence of cataract due to steroids is found to be variable in different disease conditions, sometimes the disease itself increasing the susceptibility to cataract formation (1). It is also suspected that M. leprae may contribute to cataract formation due to quinone formation (22). Eleven patients had severe impairment of vision and needed cataract surgery, 7 had intraocular lens (IOL) and 2 wear spectacles. All are able to attend to their daily work. Two patients could not subject themselves to surgery due to other family problems. Vision improved in 12 patients on reduction of the steroid dose. Aspirin and vitamin C are said to be helpful in preventing cataract formation. Hence, we now routinely add vitamin C 100 mg and aspirin 300 mg daily to the regimens of patients requiring more than 6 months of steroid therapy (5,9,36). How far this helps, we have to wait and see.
Diabetes. Steroid-induced diabetes is a well-recognized complication, and we encountered this within 3 months of starting steroid therapy. There was a wide range of postprandial blood sugar levels in these patients (from 230 mg/l00 ml and 700 mg/100 ml). We were able to control diabetes with oral sulfonyl ureas (glibenclamide). To our great relief, the diabetes was always temporary and we could always stop antidiabetic therapy after the prednisolone dosage was reduced to <30 mg/day. Fortunately, none of these patients developed permanent diabetes or ketoacidosis or coma.
Tuberculosis. Pulmonary tuberculosis is another serious complication we came cross in these patients. They started complaining of loss of appetite, loss of weight and recurrent fever and cough. A chest X-ray confirmed the disease. It occurred mostly in patients suffering from type 2 reaction. Steroids could be continued along with appropriate anti-tuberculosis therapy. Three patients (all with very severe type 2 reaction) died, probably the tuberculosis worsening the type 2 reaction or vice versa.
Osteoporosis. We do not have facilities for assessing bone mineral density. Hence, the incidence of this complication is only an inference based on clinical events which occurred in those patients requiring more than 6 months of steroid therapy. They were all managed conservatively. The following events were observed: hip fracture, 1 (postmenopausal, type 2 reaction); tarsal disintegration, 2 (both type 1 reaction with foot drop - could denervation also have played a part?); and lumbar pain (vertebral osteoporosis?), 10 (all type 2 reaction).
Oral supplements of calcium help to maintain bone density to some extent (25). Hence, we add oral calcium tablets to the regimens of patients who are on steroids for more than 6 months. Edidronate and other biphosphonates may be useful supplements, but their cost is prohibitive (26). The usefulness of cyclosporin and other drugs have to be investigated further (16).
DISCUSSION
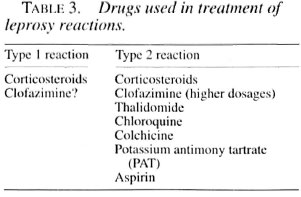
Many drugs have been used in the treatment of leprosy reactions in addition to specific antileprosy chemotherapy (Table 3). Corticosteroids are effective in both types of reactions (3,13,20) . Even though thalidomide gives excellent relief in type 2 reaction, it is not easily available, cannot be given to women of child-bearing age, and for men it has to be given under supervision after hospitalization, hence its continued administration over a long period is not practicable (24,34). Other drugs for type 2 reaction are slow in action and not very effective in severe degrees of prolonged reactions (10,12,15,21,23,32), Hence, using corticosteroids with other drugs in various combinations and dosages becomes mandatory with the attendant adverse effects inevitable. Also, corticosteroids are the only drugs available at all places at a reasonable cost. At the same time, the awareness of the problems likely to arise because of steroid therapy and the magnitude of the problem will help the physician and other field workers engaged in leprosy therapy to approach the problem of reactions with caution and without undue prejudice toward steroids, because proper treatment of reactions will definitely prevent the majority of disabilities (27,35).
CONCLUSION
We have described the adverse effects we came across during treatment of patients suffering from leprosy reactions (type 1 and type 2) with corticosteroids with a view to present the entire problem in the proper perspective. The incidence of adverse effects is found to be more among patients suffering from type 2 reaction. This is because the type 2 reactions occurred frequently and lasted for a longer duration than did the type 1 reactions. Hence, a higher percentage of patients with type 2 reaction (230 out of 249, 92%) required steroids for 6 or more months than did the patients with type 1 reaction (266 out of 581, 45%). Corticosteroids are available easily at a reasonable cost, but we feel that efforts must be taken to identify alternative drugs of equal or higher efficacy without the side effects. Some workers have claimed the usefulness of cyclosporin, azathioprine and methotrexate but they are very costly, need more supervision, and may not be very suitable for field use. Feedback and comments from the readers on this important problem will help the leprosy workers as well as benefit leprosy patients.
Acknowledgment. I thank the administration of the Sacred Heart Leprosy Centre for permission to conduct this study. My thanks also go to all the medical officers who extended their kind cooperation. I gratefully acknowledge the cooperation of the Medical Records Department staff and the laboratory technicians while collecting this data. I thank Mr. S. Muthusamy lor secretarial assistance. Special thanks go to Mr. S. Seshadri for typing the manuscript patiently and overcoming all difficulties in spite of repeated alterations.
REFERENCES
1. Allen, M. B., Ray, S.G., Leitch, A. G., Dhillon, B. and Cullen, B. Steroid aerosols and cataract formation. BMJ 299(1989)432-433.
2. Becx-Bleumink, M. The management of nerve damage in the Leprosy Control Services. Lepr. Rev. 61(1990)1-11.
3. Becx-Bleumink, M. and Berhe, D. Occurrence of reactions: their diagnosis and management in leprosy patients treated with multidrug therapy; experience in the Leprosy Control Programme of the All Africa Leprosy and Rehabilitation Training Centre (ALERT) in Ethiopia. Int. J. Lepr. 60(1992)173-184.
4. Bjune, G. Reactions in leprosy. Lepr. Rev. Special Issue (1983) 61S-67S.
5. Cheng, H. Aspirin and cataract. Br. J. Ophthalmol.76(1992)257-258.
6. Daniel E., Alexander, R. and Chacko S. Ocular leprosy: do steroids complicate matters? (Letter) Int. J. Lepr.63(1995)115-117.
7. Groenen, G., Janssens, L., Kayembe, T, Nollet, E., Coussons, L. and Pattyn, S. R. Prospective study on the relationship between intensive bactericidal therapy and leprosy reactions. Int. J. Lepr. 54(1986)236-244.
8. Guplandi, M. and Tittobello, A. Steroid ulcers: a myth revisited. BMJ304(1992)655-656.
9. Harding, J. J. and Van Heningen, R. Drugs, including alcohol, that act as risk factors for cataract and possible protection against cataract by aspirinlike analgesics and cyclopenthiazide. Br. J. Ophthalmol.72(1988)809-814.
10. Hastings, R. C. and Trautman, J. R. B.633 in lepromatous leprosy; effect in erythema nodosum leprosum. Lepr. Rev.39(1968)3-7.
11. Haynes, R. C, Jr. Adrenocorticotropic hormone; adrenocortical steroids and their synthetic analogs; inhibitors of the synthesis and actions of adrenocortical hormones. In: Goodman and Oilman's The Pharmacological Basis of Therapeutics. 8th edn. Gilman, A. G., Rall, T. W., Nies, A. S. and Taylor, P., eds. New York: Pergamon Press, 1990, pp. 1431-1462.
12. Imkamp, F. M. J. H. Clofazimine (Lamprene or B633) in lepra reactions. Lepr. Rev.52(1981)135-140.
13. Jacobson, R. R. Treatment of leprosy. In: Leprosy. Hastings. R. C, ed. Edinburgh: Churchill Livingstone, 1985, pp. 211-217.
14. Jopling, W. H. Side effects of antileprosy drugs in common use. Lepr. Rev.54(1983)261-270.
15. Karat, A. B. A., Jeevaratnam, A., Karat, S. and Rao, P. S. S. Efficacy of clofazimine in the prophylaxis and suppression of reactive phases of lepromatous leprosy. Int. J. Lepr.39(1971)838-841.
16. Kelly, P. J., Sambrook, P. N. and Etsman, J. A. Potential protection by cyclosporin against glucocorticoid effects on bone. (Letter) Lancet 2(1989)1388.
17. Mshana, R. N. Erythema nodosum leprosum is precipitated by an imbalance of T lymphocytes. Lepr. Rev.53(1982)1-7.
18. Naafs, B., Pearson, J. M. H. and Wheate, H. W. Reversal reaction: the prevention of nerve damage; comparison of short- and long-term steroid treatment. Int. J. Lepr.47(1979)7-12.
19. Naafs. B. and Wheate, H. W. The time interval between the start of antilcprosy treatment and the development of reactions in borderline patients. Lepr. Rev. 49(1978) 153-157.
20. Pearson, J. M. H. The use of corticosteroids in leprosy. Lepr. Rev. 52(1981)293-298.
21. Pfaltzgraff, R. E. The control of neuritis in leprosy with clofazimine. Int. J. Lepr.40(1972)392-398.
22. Prabhakaran, K. Cataract in leprosy: a biochemical approach. Lepr. Rev.42(1971)11-1 3.
23. Ramanujam, K. and Dhakmendra. Management of reactions in leprosy. In: Leprosy. Vol. 1. Dharmendra, ed. Bombay: Kothari Medical Publishing House, 1978, pp. 512-534.
24. Ramu, G. and Girdhar, A. Treatment of steroid dependent cases of recurrent lepra reaction with a combination of thalidomide and clofazimine. Lepr. India 51(1979)497-504.
25. Reid, D. M., Nicoll, J. J., Smith, M. A., Hig-Gings, B., Tothill, P. and Nuki, G. Corticosteroids and bone in asthma: comparison with rheumatoid arthritis and polymyalgia rhcumalica. BMJ 293(1985)1463-1466.
26. Reid, I. R., Heap, S. W., King, A. R. and Ibbertson, H. K. Two year follow-up of biphosphonate (APD) treatment in steroid osteoporosis. (Letter) Lancet 2(1988)1144.
27. Richardus, J. H. and Smith, W. C. The risk of standardised regimens of corticosteroids for the treatment of leprosy reactions in the field. Lepr. Rev.661995)328-329.
28. Ridley, D. S. and Radia, K. B. The histological course of reactions in borderline leprosy and their outcome. Int. J. Lepr. 49(1981)383-392.
29. Ridley, M. J. and Ridley, D. S.The immunopathology of erythema nodosum leprosum: the role of extravascular complexes. Lepr. Rev. 54(1983)95-107.
30. Rose, P. and Waters, M. F. R. Reversal reactions in leprosy and their management. Lepr. Rev.62(1991)113-121.
31. St. Claire Roberts, D. Steroids, the eye and general practitioner. BMJ292(1986)1414-1415.
32. Sarojini, P. S. and Mshana, R. N. Use of colchicine in the management of erythema nodosum leprosum (ENL). Lepr. Rev.54(1983)151-153.
33. Saul, A. and Segura, N. Lepra reaction: its relation with fragmentation of Mycobacterium leprae under sulfone therapy. Int. J. Lepr.34(1966)17-22.
34. Sheskin, J., Magora, A. and Sagher, F. Motor conduction velocity studies in patients with leprosy reaction treated with thalidomide and other drugs. Int. J. Lepr.37(1969)359-364.
35. Srinivasan, H. Prevention of disabilities in leprosy - a practical guide. Geneva: World Health Organization, 1993.
36. Van Heningen, R. and Harding, J. J. Do aspirinlike analgesics protect against cataract? A casecontrol study. Lancet1(1986)1111-1113.
37. Williamson, T. H., Strong, N. P., Sarrow J., Aggarwal, R. K. and Harrad, R. Contrast sensitivity and glare in cataract using the Pelli-Robson chart. Br. J. Ophthalmol.76(1992)719-722.
1. B.Sc. M.B.B.S., D.P.M., Specialist, Department of Medicine, Schieffelin Leprosy & Research Training Centre, Karigiri 632 106 N.A.A. District, Tamil Nadu, South India: fax: 91-416-74274.
Received lor publication on 5 February 1997.
Accepted for publication in revised form on 31 October 1997