- Volume 66 , Number 1
- Page: 22–8
Fractionation, characterization and diagnostic potential of circulating leprosy antigens isolated f rom sera of lepromatous leprosy patients
ABSTRACT
Circulating antigen was isolated f rom lepromatous sera by ammonium sulfate precipitation. The protein fraction between 36% and 75% ammonium sulfate was reactive with leprosy sera. Further fractionation of Ultrogel AcA 34 gel filtration column gave four protein fractions, CLA1 CLA2, CLA3 and CLA4. CLA1 and CLA2 showed antigenic activity. On SDS-PAGE analysis and elution of the protein fractions, CLA17, CLA2-1 and CLA2-7 were found to be reactive with leprosy sera. On evaluating the diagnostic utility of these fractions, CLA1-7 could detect IgG antibodies in 80% of the lepromatous (LL) and in 40% of the tuberculoid (TT) serum samples. Fraction CLA,-1 reacted with IgM antibodies in 80% of the LL and TT patients; fraction CLA2-7 reacted with IgM antibodies in 70% of the LL and TT sera. Biochemical characterization indicated that CLA1-7 was a glycoprotein while CLA2-1 and CLA2-7 were lipoproteins in nature. When tested by an inhibition ELISA, fraction CLA2-7 inhibited the binding of anticeramide antibodies to a ceramide-coated plate while thin-layer chromatography of fractions CLA2-1 and CLA2-7 showed a spot with an Rf value similar to that of standard ceramide. This study thus shows for the first time the presence of ceramide in circulating leprosy antigen.RÉSUMÉ
Un antigène circulant a été isolé de serums lépromateux par précipitation du sulfate d'ammonium. La fraction protéique entre 36% et 75% de sulfate d'ammonium réagissait avec les serums lépreux. Un fractionnement ultérieur sur colonne de filtration en gel Ultrogel Aca 34 a donné quatre fractions protéiques, CLA1, CLA2, CLA3 et CLA4. CLA, et CLA2 ont montré une activité antigénique. A l'analyse SDS-PAGE et élution des fractions protéiques, CLA1-7. CLA2-1 et CLA2-7 étaient trouvés comme réagissant avec les serums lépreux. A l'évaluation de l'utilité diagnostique de ces fractions. CLA1-7 pouvait détecter des anticorps IgG chez 80% de échantillons de scrums lépromateux (LL) et 40% des tuberculoides (TT). La fraction CLA2-l réagissait avec les anticorps IgM chez 80% des patients LL et TT; la fraction CLA2-7 réagissait avec les anticorps IgM dans 70% des serums LL et TT. La caractérisation biochimique indiquait que CLA1-7 était une glycoprotéine, tandis que CLA2-1 et CLA2-7 étaient de nature lipoprotéique. Quand elle fut testée par un ELISA d'inhibition, la fraction CLA2-7 inhibait la liaison des anticorps an'.icéramides à une plaque revêtue de céramides. tandis que la chromatographic en couche mince des fractions CLA2-1 et CLA2-7 montrait une tache avec une valeur Rf semblable à celle de la céramide standard. Cette étude montre donc pour la première fois la présence de céramides dans un antigène lépreux circulant.RESUMEN
Se aislaron los complejos inmunes circulantes de los sueros de pacientes con lepra por precipitación con sulfato de amonio. La fracción de proteínas precipitada con 36-75% de sulfato de amonio fue reactiva con los sueros de los pacientes con lepra. La separación adicional de esta fracción usando una columna de Ultrogel AcA 34 dio 4 fracciones proteicas, CLA1, CLA2, CLA3 y CLA4. CLA1 y CLA2 mostraron actividad antigénica. Por PAGE-SDS y elución, se obtuvieron varias fracciones (CLA1-7, CLA1-1 y CLA2-7) que mantuvieron su reactividad con los sueros. Al valorar la utilidad diagnóstica de estas fracciones se encontró que la fracción CLA1-7 pudo detectar anticuerpos IgG en el 80% de los sueros de los pacientes lepromatosos y en el 40% de los pacientes tuberculoids. La fracción CLA2-7 reaccionó con anticuerpos IgM en el 70% de los sueros LL y TT. La caracterización bioquímica de las fracciones indicó que CLA1-7 fue una glicoproteína y que CLA2-1 y CLA2-7 fueron lipoproteínas. En un ensayo de inhibición por ELISA se encontró que la fracción CLA2-7 inhibió el enlazamiento de anticuerpos anticerámido a placas cubiertas con cerámido, mientras que por cromatografía en capa lina, las fracciones CLA2-1 y CLA2-7 mostraron una mancha con un Rf similar al encontrado con el cerámido estándar.Este estudio muestra, por primera vez, la presencia de cerâmido entre los antïgenos que circulait en los pacientes con lepra.Leprosy is a chronic granulomatous disease primarily affecting the peripheral nerves, although other organs may also be involved. If not for the nerve pathology, leprosy would have been just another skin disease. It is thus necessary to understand the pathogenesis in leprosy. Although much work has been done on surface and sonicate antigens of Mycobacterium leprae, it will be of interest to identify the circulating antigens involved in the humoral and cell-mediated immunity in leprosy. The granuloma formed around the bacilli-infected tissue leads to inflammation and is the cause of neuritis. A novel method for the identification of these antigens would be to isolate them from the sera of M. leprae-infected individuals. Similar studies have been carried out earlier (3,10) where the fractionated circulating filarial antigens were found to be specific and useful in immunodiagnostics. Ramaprasad and Harinath (8) have isolated filarial antigens from hydrocoele fluid.
In this paper we attempted to isolate, fractionate and characterize circulating antigens in leprosy sera and also to evaluate the diagnostic utility of these fractions.
MATERIALS AND METHODS
Sera. Human sera belonging to leprosy patients and endemic normal controls were screened for this study. Twenty leprosy serum samples from untreated lepromatous leprosy patients with a bacterial index (BI) of more than 2+ were collected and pooled. Endemic normal (EN) blood samples were taken from healthy individuals living in an endemic region but having no history of leprosy. Sera were separated from blood samples and stored at -20°C after adding sodium azide (0.01%) as a preservative until use.
Isolation and fractionation of circulating leprosy antigen (CLA). The sera of 20 lepromatous leprosy patients with a BI of more than 2+ were pooled. The antibodies were precipitated with 35% ammonium sulfate, the supernatant was separated and further precipitated with ammonium sulfate (36%-75% and 75%-100%). The precipitate of each fraction was then reconstituted in a minimum volume of 0.05 M. sodium phosphate buffer (SPB), pH 7.2, dialysed overnight in the same buffer, and checked for reactivity by an indirect ELISA with the leprosy sera. The protein fraction showing reactivity with leprosy sera was then labelled as circulating leprosy antigen (CLA). The proteins were estimated by the method of Lowry, et al. (5), and were stored at -20°C with 0.01% sodium azide as preservative.
Fifty mg CLA in 0.05 M SPB, pH 7.2, was fractionated on an Ultrogel AcA-34 (Pharmacia LKB, France) gel filtration column (1.6 × 50 cm) previously equilibrated with the same buffer at 4°C. Ultrogel AcA 34 gel contains 3% acrylamide and 4% agarose and is stable in a buffer solution with ionic strength of at least 0.05 between pH 3 and pH 10. The flow rate of the elution was fixed at 8 ml/hr using a 2132 microplex peristaltic pump (LKB, Bromma, Sweden). Two ml fractions were collected using a Frac 100 fraction collector (Pharmacia, Uppsala, Sweden) while the protein concentration in the eluate was monitored at 280 nm (UV Cord II; LKB) and recorded with one channel recorder (LKB). The protein peaks obtained were pooled separately and concentrated to 1 ml by ultrafiltration at 4°C. The fractionation procedure was continued using different batches of CLA and a similar elution pattern was observed. The proteins in the four fractions (CLA1 CLA2 CLA3 and CLA4) were estimated by Lowry, et al.'s method and tested for antigen reactivity by an ELISA using pooled LL, pooled TT and pooled EN sera using antihuman IgG+ IgM horseradish peroxidase (HRPO) (Sigma Chemical Co., St. Louis, Missouri, U.S.A.) conjugate. The diagnostic utility of the antigen fractions was then evaluated by an indirect ELISA.
Production of anti-EST-11 antibodies.
Polyclonal antibodies to a crossreactive antigen (EST-11) obtained from early cultures of M. tuberculosis H37Ra as described by Lodam, et al. (4) were produced in mice and used in this assay. BALB/c mice were immunized intraperitoneally with EST-11 antigen, and the gamma globulin fraction of mouse ascitic fluid showing anti-EST-11 antibody was precipitated by 33% ammonium sulfate. The precipitate was resuspended in 0.01 M SPB (pH 7.2) and dialysed against the same buffer. After dialysis the protein was estimated by Lowry, et al.'s method.
Production of anticeramide antibodies.
Ceramide (Centre for Biochemical Technology, New Delhi, India) was conjugated to bovine serum albumin (BSA) by the mixed anhydride method (12). New Zealand white rabbits were immunized with 1 mg conjugate in Freund's complete adjuvant (Sigma). The animals were then further immunized with 500 µg antigen twice at weekly intervals. The rabbits were bled 2 weeks after the second booster dose. Ceramide specific antibodies were isolated from the antiserum by adsorbing it with BSA (1). Anticeramide antibodies were then separated by precipitation with ammonium sulfate.
Indirect ELISA. An optimal concentration of CLA fraction (250 ng/50 µl/well) was coated onto flat-bottom, polystyrene microtiter plates (Tarson Products, India) and incubated at 4°C overnight. The plates were then washed with phosphate buffer saline (PBS) 0.05 M with 0.05% Tween (PBS/T) and further incubated with 100 pi volume of 3% BSA (Lupin Labs, India) for 1 hr at 37°C. Sera were diluted 1:300 in PBS/T and incubated at 37°C for 1 hr. The binding was then probed by incubating the plates with antihuman IgG (1:2000) or IgM (1:3000) HRPO conjugate at 37°C for 1 hr. The reaction was visualized by the addition of 50 µl of orthophenylene diamine (OPD; Sigma) substrate, consisting of 5 mg OPD, 5 ml citrate phosphate buffer pH 5.0 and 5 µl 30% H202, and blocked by the addition of 2 N HC1. the optical density (OD) was read in an ELISA reader (Dynatech MR 250; Dynatech Labs. Inc., Chantilly, Virginia, U.S.A.) at 490 nm.
Inhibition ELISA. Optimal concentrations of nerve/bacterial antigen (ceramide; Centre for Biotechnology and S100; Sigma) 100 ng/50 µl/well and EST-11 500 ng/50 µl/well were coated onto flat-bottom polystyrene plates and incubated at 37°C for 3 hr. After one washing 3% BSA was added to the wells and incubated at 37°C for 2 hr; 100 pi (5 pg/ml) of reactive circulating antigen (CLA,-7, CLA:-I or CLA,-7) was then reacted with respective anti-EST-11, anti-SlOO or anticeramide antibodies (500 ng in 10 pi) and incubated at 37°C for a half hour. The supernatant after centrifugation was added to the sensitized plate and incubated for 1 hr at 37°C. Optimally diluted antirabbit HRPO (Cappel, West Chester, Pennsylvania, U.S.A.) or antimouse HRPO (Lupin) were added to the plates and the reaction was visualized using OPD substrate.
Fractionation of CLA by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). CLA1 and CLA2 were resolved by SDS-PAGE on 10% nongradient slab gel (12 × 12 cm) and sliced horizontally at 1-cm intervals (12 slices designated CLA,-1 to 12 and CLA2-1 to 12), and the proteins in each slice were electroeluted. The eluates were then used to check the reactivity with leprosy sera by an indirect ELISA.
Identification of bacterial/nerve antigens in CLA. The presence of bacterial or nerve antigens in the circulating antigen fractions was studied by an inhibition ELISA.
Biochemical characterization. The enzymes alpha-amylase (Sigma); chymotrypsin (SD Fine Chemicals Ltd., India); trypsin (SRL, India); promise (Boehringer GmbH, Mannheim, Germany) and lipase (SRL) (4 mg each) were coupled separately to 1 ml CNBr Sepharose 4B beads (Pharmacia Fine Chemicals) (7). The protein in the supernatant was estimated before and after coupling, and the binding obtained was 80% - 85% in all cases.
Enzyme treatment. CLA1-7, CLA2-1 and CLA2-7 fractions were treated separately with different enzyme coupled beads and the mixture kept at 37°C for 24 hr. The supernatant was separated by centrifugation at 4°C and diluted optimally for use in an indirect ELISA.
Heat inactivation. Antigens were heated at 100°C for 30 min in a water bath (9). After cooling, fractions were centrifuged and the supernatants were used in an indirect ELISA.
Periodate treatment. The three antigens were treated separately with 0.1 M sodium metaperiodate (BDH, England) at 37°C for 24 hr (6). The reaction was stopped by dialysis against 0.01 M sodium phosphate buffer pH 7.2 after which the antigens were used in an indirect ELISA.
Thin-layer chromatography. Thin-layer chromatography of neutral glycosphingolipids was performed as described by Skipski (11), stained with a sodium hypochlorite-benzidine spray to identify sphingolipids, and the pattern was compared with that of standard ceramide.
RESULTS
The results of the analysis of fractionated sera showed antigenic activity in the 36% - 75% ammonium sulfate fraction. This antigenic fraction was labelled as circulating leprosy antigen (CLA). Fractionation of CLA on an AcA-34 gel filtration column resulted in four protein peaks, CLA1, CLA2, CLA, and CLA4 (Fig. 1). The proteins in the peaks were concentrated and tested for reactivity with the leprosy sera. By indirect ELISA, fractions CLA, and CLA, were reactive with the leprosy sera (The Table).
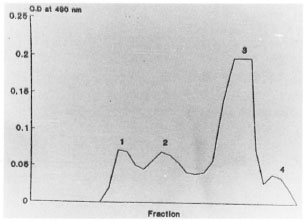
Fig . 1. Fractionation of circulating leprosy antigenon AcA-34 gel filtration column.

While fraction CLA1 reacted with IgG antibodies, CLA, showed the presence of IgM antibody in leprosy sera. On analysis by SDS-PAGE, CLA1, gave 17 protein bands, while the CLA2 fraction gave 21 protein bands in the molecular weight range of 12,000 to 120,000 Da. On testing the 12 eluates obtained after electroelution of the CLA resolved gel by an indirect ELISA, fraction CLA1-7 reacted with pooled leprosy sera while with the CLA2 resolved gel, fractions CLA2-1 and CLA,-7 were reactive.
The diagnostic use of the three reactive fractions (CLA1-7, CLA,-1 and CLA2-7) for the detection of antibody in leprosy sera was studied by an indirect ELISA. CLA1-7 detected IgG antibodies in 80% of the LL and 40% of the TT patients, fraction CLA2-1 detected IgM antibodies in 80% of the LL and 80% of the TT patients, and the CLA2-7 fraction reacted with IgM antibodies in 70% of the LL and TT patients (Fig. 2a-c).
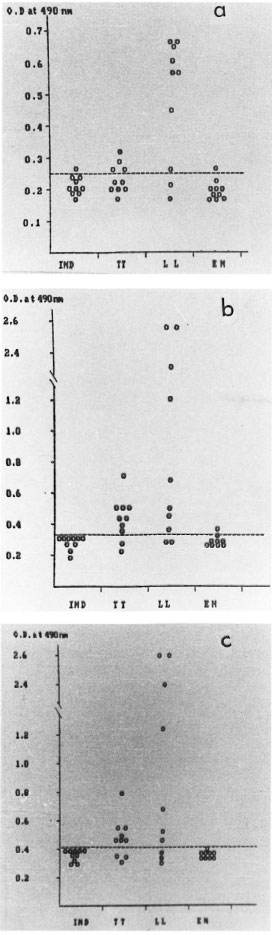
Fig . 2. Scattergram showing seroreactivity with leprosy sera to: A) CLA1-7 (IgG antibodies); B) CLA2-I(IgM antibodies); C) CLA2-7 (IgNI antibodies).
In an inhibition ELISA using the three reactive antigen fractions, fraction CLA2-7 inhibited the binding of anticeraniide antibody to ceramide coated onto the PVC microliter plates, but did not inhibit the binding of anti-EST-11 antibody to EST-11 or anti-S-100 antibody to S-100 coated onto the plate. Fractions CLA1-7 and CLA2-1 did not inhibit the binding of anticeramide antibody, anti-EST-11 antibody, or anti-S-100 antibody to the plates. Fig. 3a-c shows the effect of heat, chymotrypsin, trypsin, pronase, periodate and lipase action on CLA1-7, CLA2-1 and CLA2-7. The antigenicity of CLA1-7 was lost by treatment with heat, proteolytic enzymes and periodate, while no loss of activity was seen after lipase treatment. CLA2-1 was heat stable and the reactivity was lost after treatment with proteolytic enzymes and lipase. No loss of reactivity was seen after periodate oxidation. After separation by thin-layer chromatography and staining for sphingolipids, CLA2-1 showed the presence of ceramide (Fig. 4). The CLA2-7 fraction was also heat stable, and loss of reactivity was observed after treatment with lipase and chymotrypsin. Antigen activity was not affected after periodate treatment. On separation by thin-layer chromatography and staining for sphingolipids, CLA,-7 showed the presence of ceramide.
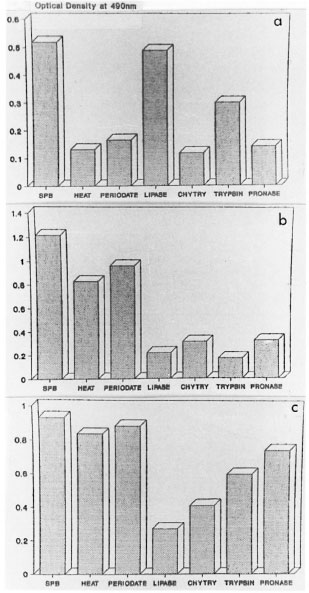
Fig . 3. The effect of heat, periodate and enzymedigestion on the reactivity of: A) CLA1-7; B) CLA2-1 C) CLA2-7. SPB = sodium phosphate buffer; heat =100°C × 30 min, chytry chymotrypsin.
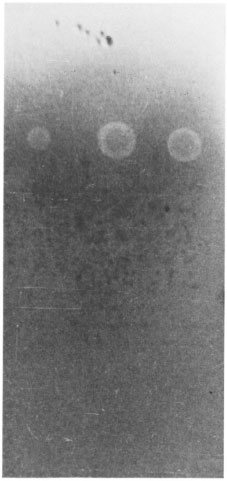
Fig . 4. Thin-layer chromatography of CLA2- 1 and CLA2-7 antigen fractions showing the presence of ceramide.
DISCUSSION
The isolation of circulating antigens in sera from individuals suffering from different parasitic and other infectious diseases has been explored in the past (2,3), and some of these antigens were also studied for their diagnostic utility (3,10). The ammonium sulfate fraction followed by AcA 34 gel filtration column chromatography yielded two active fractions, CLA1 and CLA2, with preferential reactivity to IgG and IgM antibodies, respectively.
On analysis with SDS-PAGE eluates of fractions CLA1 and CLA2 by an indirect ELISA, three reactive fractions (CLA1-7, CLA2-1 and CLA2-7) were identified. Of these three fractions CLA2-1 and CLA2-7 could detect 80% and 70% of LL leprosy sera screened, thus demonstrating the diagnostic value of these fractions. Further extensive studies are warranted to explore the diagnostic potential in different groups of leprosy sera.
Biochemical characterization showed that CLA1-7 was found to be a heat-labile glycoprotein, while fractions CLA2-1, and CLA2-7 were heat-stable lipoproteins (Fig. 3a-c). Fraction CLA2-7 was found to inhibit the binding of anticeramide antibody to a ceramide-coated plate, demonstrating the presence of ceramide in this fraction. No such inhibition was observed with fractions CLA1-7 and CLA2-1. However, fractions CLA2-7 and CLA2-1 stained for sphingolipids by thin-layer chromatography and showed an Rf value similar to that of standard ceramide. The nonreactivity of CLA2-1 compared to CLA2-7 with antibovine brain ceramide antibody may possibly be due to the absence of the crossreactive epitope in CLA2-1.
As far as we are aware, this is the first study to show the diagnostic potential of circulating leprosy antigen in sera of patients and the presence of cermidc in circulating antigen.
Acknowledgment. The authors are grateful to Dr. Sushila Nayar, Director, Kasturba Health Society; Shri Dhirubhai Mehta, Vice President, and Dr. O. P. Gupta. Dean. MGIMS. for the keen interest and encouragement given to complete this work. This work was done under the TDRC project of MGIMS.
REFERENCES
1. Avrameas, S. and Ternynck, T. The cross linking of proteins with glutaraldehyde and its use lor the preparation of immunoadsorbants. Immunochemistry 6(1969)53-56.
2. Harinath. B. C. Detection and diagnostic utility of in vitro and in vivo released antigens in bancroftian lilariasis. J. Comm. Dis. 18(1986)261-266.
3. Kaliraj, P., Ghirnikar, S. N. and Harinath, B. C. Detection of circulating filarial antigen in bancroftian filariasis. Indian J. Exp. Biol. 17(1979)1148-1149.
4. Lodam, A. N., Reddy, M. V. R., Narang, P., Gupta, O. P. and Harinath, B. C. Fractionation, analysis and diagnostic utility of Mycobacterium tuberculosis H37Ra excretory-secretory antigen in pulmonary tuberculosis. Indian J. Biochem. Biophys. 33(1996)66-71.
5. Lowry, O. H., Rosbrough, N. J., Farr, A. L. and Randell, R. J. Protein measurement with the Folin-phenol reagent. J. Biol. Chem. 193(1951)265-275.
6. Mok, W. Y., Buckley, H. R. and Campbell, C. C. Characterization of antigens front Type A and B yeast cells of Histoplasma capsulatum. Infect,Immum. 16(1977)461-466.
7. Ramaprasad, P. and Harinath, B. C. Fractionation and characterization of urinary filarial antigen in bancroftian filariasis. Asian Pacific J. Allergy Immunol. 5(1987)173-178.
8. Ramaprasad, P. and Harinath, B. C. Fractionation, characterization and diagnostic potential of filarial antigens isolated from hydrocoele fluid in bancroftian lilariasis. Trans. R. Soc. Trop. Med. Hyg. 83(1989)90-94.
9. Reddy, M. V. R., Malhotra, A., Prasad, G. B. K. S. and Harinath, B. C. Evaluation of fractionated Wuchereria bancrofti microfilarial excretory secretory antigen for diagnosis of bancroftian filariasis by enzyme linked itnmunosorbant assay. J. Biosci. 6(1984)165-171.
10. Reddy, M. V. R., Prasad, G. B. K. S. and Harinath, B. C. Isolation and evaluation of antigens from microfilaraemia plasma and immune complexes for diagnosis of bancroftian lilariasis. Indian J. Pathol. Microbiol. 2(1986)179-188.
11. Skipski, V. P. Thin layer chromatography of neutral glycosphingolipids. Methods in Enzymology Vol. 35 Part B. Lowenstein, J. M., ed. New York: Academic Press, 1975, pp.396-425.
12. Thorell, J. I. and Larson, S. M. Radioimmunoassay and Related Techniques: Methodology and Clinical Applications. St. Louis: Mosby Publications, 1978, pp. 258-259.
1. M.Sc, Department of Biochemistry.
2. M.D., Division of Skin and V.D., Department of Medicine.
3. Ph.D., Director Professor and Head, Department of Biochemistry &. J.B. Tropical Disease Research Center, Mahatma Gandhi Institute of Medical Sciences. Sevagram 442 102. M.S., India.
Reprint requests to Professor Harinath.
Received lor publication on 4 September 1997.
Accepted lor publication in revised form on 12 December 1997.