- Volume 66 , Number 2
- Page: 125–30
Does MDT arrest transmission of leprosy to household contacts?
ABSTRACT
The multidrug therapy program with the World Health Organization (WHO)-recommended treatment (WHO/MDT) regimens has given the hope of early case detection and rendering a leprosy patient, especially a multibacillary (MB) patient, noninfectious within a short period of time. Hence, the duration of exposure for household contacts to infection is expected to be remarkably less when compared to exposure to MB leprosy patients on dapsone monotherapy. A total of 1661 household contacts of skinsmear-positive leprosy patients were recorded f rom 1984 to 1994. Follow up of these individuals [8403 person-years at risk (PYR)] revealed that the incidence of leprosy was 7.7 per 1000 PYR, which was 8 times more than that of the general population. The risk was more if there was a coprevalent case in the family. The incidence of leprosy declines f rom the third year of surveillance onward, and declines more so in children. Although disease transmission should have been arrested as soon as the index case was started on MDT, the incidence of leprosy among the household contacts was still high when compared to that of the total population. Effective intervention needs to be introduced to reduce the risk of contacts developing leprosy.RÉSUMÉ
Le plan d'administration de polychimiothérapie (PCT) recommendé par l'Organisation mondiale de la Santé (PCT/OMS) a apporté l'espoir d'une détection plus précoce des nouveaux cas et de rendre un patient atteint de lèpre, en particulier les patients multibacillaires (MB), non-infectieux dans un court lapse de temps. Ainsi, la durée de contagion vis à vis des personnes en contact avec le patient devrait être beaucoup plus courte en comparaison du temps de contagion vis à vis de patients MB en monothérapie (Dapsone). Un nombre total de 1661 personnes en contact, dans la même maisonnée, avec des patients lépreux présentant un frottis de SUO dermique positif, fut enrôlé et suivi de 1984 à 1994. Le résultat du suivi de ces individus ¡8403 personnes-années à risque (PAAR)| a révélé une incidence de lèpre de 7,7 pour 1000 PAAR, ce qui est 8 fois plus que celle de la population générale. Le risque était plus élevé si il y avait présence d'un cas co-prévalent dans la famille. L'incidence de lèpre décline à partir de la troisième année de suivi épidémiologique, et diminue plus encore chez les enfants. Bien que la transmission de la maladie aurait dû être stoppée dès la mise sous PCT du cas index, l'incidence de la lèpre parmi les personnes contactes dans la même maisonnée étiat encore élevée lorsque comparée à la population totale. Des mesures d'intervention prophylactiques efficaces doivent être prises, afin de réduire le risque pour les personnes contacted de développer la lèpre.RESUMEN
La poliquimioterapia recommendada por la Organización Mundial de la Salud (l'QT-OMS) ha dado nuevas esperanzas para la delección temprana de los casos y para la conversión de los pacientes multibacilares (MB) en pacientes no infecciosos en un periodo corto de tiempo. Reconociendo la efectividad de la PQT es esperable que el riesgo de contagio de los contactos familiares de los pacientes tratados con PQT sea marcadamente menor que el riesgo de contagio de los contactos que conviven con pacientes MB tratados sólo con monoterapia con dapsona. Entre 1984 y 1994 se reunió un total de 1661 contactos familiares de pacientes bacilíferos. El seguimiento de estos individuos (8403 persona-años en riesgo, PYR) reveló que la incidencia de la lepre fue de 7.7 por 1000 PYR, lo cual fue 8 veces mayor que en la población general. El riesgo fue mayor cuando hubieron casos co-prevalentes en la familia. La incidencia de lepra disminuyó a partir del tercer año de seguimiento y la disminución fue más marcada en los niños. Aunque la transimisión de la enfermedad debía haberse detenido tan pronto como el caso índice hubiera sido tratado con PQT, la incidencia de la lepra entre los contactos familiares fue aun alta en comparación con la incidencia encountrada en la población general. Es evidente que se requieren medidas efectivas para reducir el riesgo de que los contactos desarrollen la enfermedad.The general population in a leprosy-endemic region is at high risk of being exposed to an infection with Mycobacterium leprae. The risk of infection is even higher among contacts of leprosy patients (1, 2). The risk of developing clinical leprosy is also high among household contacts, particularly among household contacts of a multi-bacillary (MB) patient. The incidence of leprosy among household contacts of MB leprosy patients on dapsone monotherapy ranged from 9.5 per 1000 person-years (PYR) of exposure to as much as 29.5% of contacts (3-5). It has been thought that the infectivity of MB patients on dapsone monotherapy declines slowly because dapsone is thought to be a slow-acting, bacteriostatic drug against M. leprae. In contrast, the World Health Organization (WHO)-recommended multidrug therapy (WHO/ MDT) includes rifampin, which is said to be a rapidly acting bactericidal drug capable of killing 99.9% of viable M. leprae even with a single dose. Thus, the infectivity of a MB patient is expected to decline rapidly to a negligible level after starting MDT. This would be expected to eliminate disease transmission from the MB patient to household contacts after the index case has started WHO/MDT. Is this expectation reflected in reality?
We present the findings of disease transmission to household contacts of MB leprosy patients before and after the MB patients were put on WHO/MDT. WHO/MDT reduces, but by no means eliminates disease transmission to household contacts.
MATERIALS AND METHODS
.The WHO/MDT program was introduced into the Gudiyatham project area of the Schieffelin Leprosy Research and Training Centre (SLRTC), Karigiri, India, in 1982, and the fixed-duration therapy (FDT) for MB leprosy was introduced in January 1984. The annual new case-detection rate in the project area was 1.2 cases per 1000 population, and the incidence rate from sample surveys was 0.9 cases per 1000 population. During the period from January 1984 to December 1994, 360 MB leprosy cases (skin-smear positive for acid-fast bacilli, previously untreated) were registered for treatment at the field clinics of the leprosy control unit of SLRTC. These patients were treated with WHO/MDT for 2 years. As soon as a new case was registered, all of the household contacts were physically examined for clinical signs of leprosy, and they were re-examined at annual intervals thereafter.
Definitions
Index case. A new case of MB leprosy who was started on FDT WHO/MDT during the study period.
Incident case. A new case of leprosy who was found to be healthy in the previous survey(s) was considered an incident case.
Co-prevalent case. A member of the household who had leprosy (treated in the past or currently receiving treatment) at the time of detection of the MB case (index case).
Original cohort. The household contacts recorded during the contact survey at the time of registration of the MB case (index case).
Additional cohort. The individuals who joined the household after the completion of the first contact survey, i.e., individuals who joined the household after the index case was already under treatment with FDT WHO/MDT.
Data on contact surveys were entered into a computer and statistical analysis was performed using SPSS and Epi Info (version 5). The household contacts were analyzed by grouping the data into two categories. The first category consisted of the original cohort, i.e., those who shared similar factors for exposure to leprosy infection, probably from the index case before treatment with WHO/MDT was initiated. The second category consisted of the additional cohort, i.e., those individuals who joined the family after the index case was started on WHO/MDT when the risk of exposure to infection from the index case would be expected to have been minimized. Any other history of contact with known leprosy patients prior to joining the household of the index case was not known in this group.
RESULTS
Out of 360 index cases, 22 had no household contacts and 1 family migrated out of the control area within 1 year, i.e., before any follow up, leaving a total of 337 house-holds for study. A total of 1094 household contacts (original cohort) were registered for the 337 MB patients (index cases), and 567 household contacts (additional cohort) were added during the subsequent period of surveillance. Among the total contacts 54.4% were males and 44.6% were children (less than 14 years of age).
Overall, 3.9% of the household contacts developed leprosy, i.e., were incident cases (Table 1). Among the original cohort, 55 incident cases were detected (5.0% of the original cohort). Ten incident cases were detected in the additional cohort (1.8% of the additional cohort) (Table 2). The original cohort had a higher risk of developing leprosy than the additional cohort [relative risk (RR) = 2.85, p = 0.001].

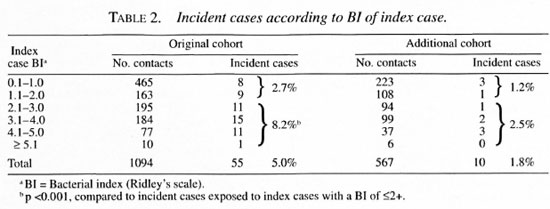
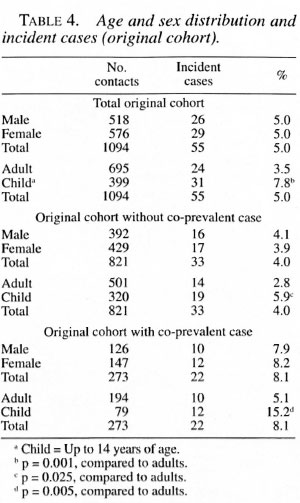
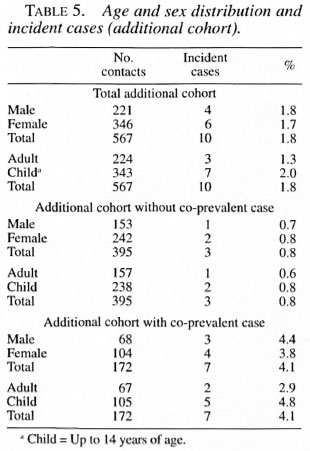
Overall, among the children 5.1% became incident cases and among the adults 2.9% became incident cases. There was no statistically significant difference in the attack rates by gender (Tables 4 and 5). Among the 337 families, 68.5% did not have any new cases. The development of incident cases in families with co-prevalent cases was twice that of families without a co-prevalent case (Table 1).
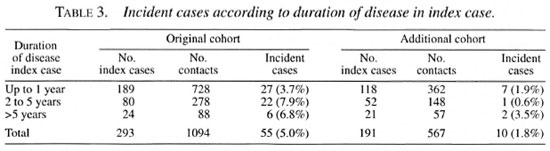
When the bacterial index (BI) of skin smears of the index case was >2+ on the Ridley scale, the relative risk in the original cohort was 3.01 compared to contacts of index cases with a BI of <2+ (Table 2).
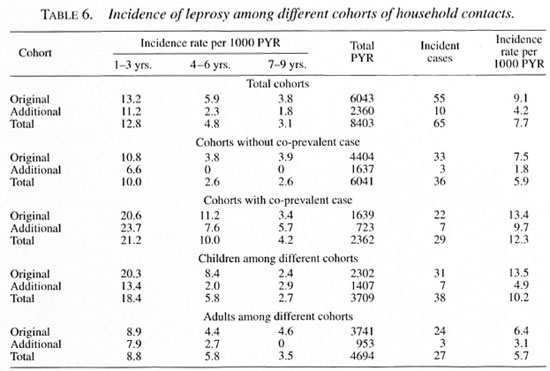
When the duration of disease in the in-dex case was more than 1 year, the attackrates in the original cohort doubled com-pared to contacts exposed to an index casewhose disease duration was less than 1 year(Tables 3 and 8).
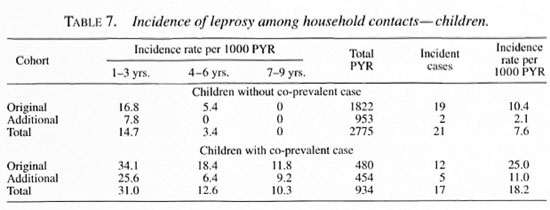
Compared to the incidence rate in thegeneral population of this area of 0.9 per1000 PYR, the incidence rate among theoriginal cohort was 9.1 per 1000 person-years of observation (PYR) and that of theadditional cohort was 4.2 per 1000 PYR.The presence of a co-prevalent case in the household was associated with an increasein the incidence among the original cohortfrom 7.5 to 13.4 per 1000 PYR and an in-crease in the incidence among the addi-tional cohort from 1.8 to 9.7 per 1000 PYR(Table 6).
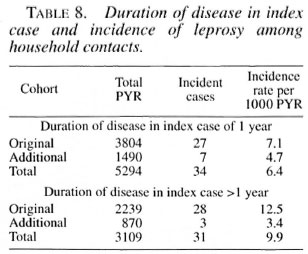
Incidence rates were highest amongyounger children in both the original andthe additional cohorts (Table 7).
In summary, we have observed that theincidence of leprosy among household con-tacts of skin-smear-positive MB cases iseight times more than in the general population. Incidence among the original cohortwas nine times more than that in the general population, and incidence among the additional cohort was more than four times that of the general population. If more than one person in the household had leprosy then the incidence is 13 times higher than that in the general population. The incidence among contacts was high during the first 3 years of surveillance, after which it declines sharply, especially in children. Incidence was twice as high among children as adults. Incidence increased among household contacts if the duration of disease in the index case was greater than 1 year, and it was higher if the index case had a BI of >2+.
DISCUSSION
In 1975, Karat, et al. (5) observed an incidence of 9.5 per 1000 PYR in this project area (Gudiyatham) among household contacts of midborderline/lepromatous patients when the standard therapy was dapsone monotherapy. This is comparable to the overall incidence of 7.7 per 1000 PYR among household contacts in the present study 2 to 12 years after the introduction of WHO/MDT.
The infectivity of the index case is expected to cease at once when the patient is started on WHO/MDT. Thus, if the index case is the direct source of the infective M. leprae, and if WHO/MDT, containing rifampin, renders the index case noninfectious, then individuals joining a household after the index case has been started on WHO/MDT (the additional cohort) should have the same risk of contracting leprosy as the general population. What we have observed is that they are over four times more likely to contract leprosy than the general population. When there is a co-prevalent case in the same household the incidence among the additional cohort increases to 10 times that of the general population, even though the co-prevalent case has either been treated successfully or is on effective treatment. Does this mean that the source of the infectious M. leprae is not the index case directly but, rather, the environment of the household?
The incidence among household contacts of MB patients in the present study was 7.7 per 1000 PYR. However, it declined from 14.3 per 1000 PYR in the first year of surveillance to 3.8 per 1000 PYR during the 7th-9th years of surveillance. This implies that treatment of the index case renders the household less infectious over time, but the effect is by no means immediate, as one would expect if the index case is the sole source of the infectious M. leprae in the household.
As a practical matter, household contacts are a high-risk group for infection by M. leprae and for the development of clinical leprosy. In the absence of a vaccine for immunoprophylaxis, consideration should be given for chemoprophylaxis. This might be more effective for eliminating leprosy than doing nothing beyond putting the index case on appropriate treatment. Putting the index case on WHO/MDT does not eliminate the transmission of the disease within a household. Other interventions are needed to minimize the risk of leprosy among household contacts.
Acknowledgment. The field study of fixed-duration therapy in multibacillary leprosy was supported by the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR). We thank Mr. P. Samuel. Project Coordinator, for his assistance in data processing. We also thank our field staff and patient families for their cooperation.
REFERENCES
1. GONZALKZ-ABRKU, E., MOIRA, N., PEREZ, M., PEREIRA, M., PEREZ, J. and GONZALEZ, A. Serodiagnosis of leprosy patients' contacts by enzyme-linked immunosorbant assay. Lepr. Rev. 61(1990)145-150.
2. GRAY, H. H. and DREISBACH, J. A. Leprosy among foreign missionaries in northern Nigeria. Int. J. Lepr. 29(1961)279-290.
3. JESUDASAN, K., BRADLEY, D., SMITH, P. G. and CHRISTIAN, M. Incidence rates of leprosy among household contacts of primary cases. Indian J. Lepr. 56(1984)600-614.
4. JESUDASAN, K., BRADLEY, D., SMITH, P. G. and CHRISTIAN, M. Time trends in the analysis of incidence rate of leprosy among household contacts. Indian J. Lepr. 56(1984)792-806.
5. RAO, P. S. S., KARAT, A. B. A., KALIAPERUMAL, V. G. and KARAT, S. Transmission of leprosy within the household. Int. J. Lepr. 43(1975)45-54.
1. P. Vijayakumaran. M.B.B.S., D.P.H., Epidemiologist.
2. K. Jesudasan, M.B.B.S., D.T.P.H., Ph.D., Epidemiologist and Head of Department.
3. N. Mani Mozhi, M.B.B.S., D.H.E., Specialist.
4. J. D. Raja Samuel, M.Sc, D.H.S., Jr. Programmer, Branch of Epidemiology and Leprosy Control, Schieffelin Leprosy Research & Training Centre, Karigiri 632 106, N.A.A. District, South India.
Received for publication on 20 April 1998.
Accepted for publication in revised form on 18 May 1998.