- Volume 66 , Number 1
- Page: 62–5
Comparison of pentoxifylline, thalidomide and prednisone in the treatment of ENL
To the Editor:
Erythema nodosum leprosum (ENL) is an inflammatory reaction that occurs in approximately 40%-50% of lepromatous leprosy patients, most commonly during treatment with antileprosy drugs. ENL is characterized by the appearance of painful, erythematous, subcutaneous nodules which are tender to the touch. These lesions are not necessarily associated with pre-existing leprosy lesions. Systemic manifestations including fever, malaise, lymphadenopathy, neuritis, and arthralgia are often observed. It appears that the pro-inflammatory cytokine tumor necrosis factor-alpha (TNF-α) may play an important role in the development of this syndrome since high plasma levels of TNF-α are found in patients during episodes of active ENL (10). Moreover, treatment of ENL patients with thalidomide alleviates the clinical symptoms concomitant with a reduction in plasma TNF-α levels (8). In vitro, thalidomide selectively inhibits the production of TNF-α by lipopolysaccharide-stimulated monocytes (6,9).
Although thalidomide is the drug of choice for the treatment of ENL (3,7,12,13) it is a potent teratogenic drug (4,5) and is not safe in women of child-bearing potential. Glucocorticoids, a family of drugs known to inhibit cytokine production by leukocytes, are also used for the treatment of ENL. Unfortunately, the prolonged use of these drugs is associated with toxicities, including immunosuppression. In an attempt to identify other treatments for ENL, alternative TNF-α inhibitors are under consideration. One such drug is pentoxifylline, a methylxanthine derivative which has been used clinically for intermittent claudication. The drug has been shown to inhibit TNF-α production in vitro (2,14) and in vivo (1,8).
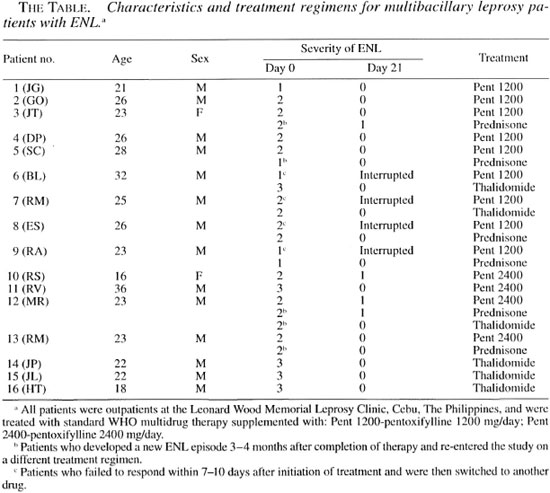
We have now tested whether pentoxifylline if effective in alleviating the signs and symptoms of ENL and have compared the efficacy of the drug in controlling the symptoms and dermatologic manifestations of ENL to the efficacy of thalidomide and of steroids. Sixteen multibacillary leprosy patients with ENL were graded for severity of disease symptoms at the start of the study and weekly thereafter (The Table). Plasma was collected at baseline and weekly for cytokine evaluation, and biopsies of ENL lesions were taken at baseline and day 2 for histologic evaluation of the response in the skin.
RESULTS AND DISCUSSION
Clinical response. The manifestations of ENL were graded for severity of disease symptoms and to enable us to evaluate the therapeutic responses, according to the outline in the legend of The Figure. Of the 16 ENL patients included in the study, nine patients received 1200 mg/day of pentoxifylline (The Table). The mean grade of ENL for these patients was 1.7 ± 0.5 (mean ± S.D.).
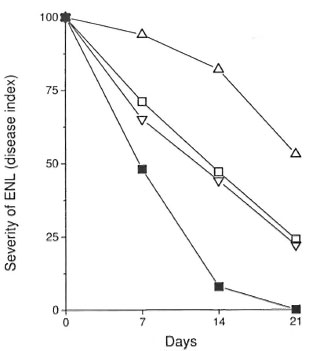
The Figure: Clinical response of ENL patients tothe different therapeutic regimens. Manifestations of ENL were graded for severity of disease symptomsand to evaluate the therapeutic responses, according tothe following criteria: Grade 0 = no ENL lesions;grade 1 = patients with <10 ENL lesions, usually with-out fever or other symptoms (these lesions were usually discovered during routine physical examination); grade 2 = patients with 10 to 20 ENL lesions who presented with mild fever, recurrent ENL, and often mildneuritic pain: grade 3 = patients with >20 ENL, nodules, lesions with blebs or pustules or ulcers, daily fevers and other symptoms such as headaches, myalgia and anorexia. Results are mean grade of ENL observedin the patients evaluated during therapy expressed as percentage (day 0 or time of diagnosis = 100%). ∆= response to 1200 mg/day of pentoxifylline; ∇= response to 2400 mg/day of pentoxifylline;  = response of patients treated with prednisone;
= response of patients treated with prednisone;  = response of patients treated with thalidomide.
= response of patients treated with thalidomide.
Five out of the nine patients treated with 1200 mg/day of pentoxifylline responded to the treatment with clinical improvement and relief of symptoms such as fever, headaches and joint pain by 2 weeks (The Figure). However, flattening or clinical clearance of the ENL skin lesions was observed only during the third week of therapy. Despite clinical improvement in response to treatment with 1200 mg/day of pentoxifylline, the skin lesions of some patients did not disappear or appeared even worse histologically with increased inflammatory infiltrate and a thicker epidermis. In the four patients who did not respond to the regimen of 1200 mg/day pentoxifylline, treatment was interrupted because the patients developed new ENL lesions with worsening of symptoms (The Table).
To evaluate whether the lack of response to 1200 mg/day of pentoxifylline therapy could be overcome by a higher dose of the drug, four new patients (patients 10 to 13) with a mean ENL severity of 2.3 ± 0.5 were treated with 2400 mg/day of pentoxifylline. By 4 to 5 days of therapy these patients reported subjective improvement of clinical symptoms, and by day 14 there was improvement of the ENL lesions. However, at day 21, two of these patients still had grade 1 ENL lesions, although they manifested no systemic clinical symptoms (The Figure).
To compare the patient response to pentoxifylline with the patient response to thalidomide, six patients were treated with thalidomide. Patients received 300 mg/day of thalidomide for the first 7 days followed by 200 mg/day of thalidomide for 7 days followed by 100 mg/day of thalidomide for the last 7 days, a total of 21 days of treatment. Three patients (patients 14-16) were entered directly into the thalidomide treatment group, two patients (patients 6 and 7) were shifted to this drug because they failed to respond to treatment with 1200 mg/day of pentoxifylline, and one patient (patient 12) suffered a relapse of ENL after successful completion of therapy with 2400 mg/day pentoxifylline (The Table; The Figure). The mean grade of ENL for these patients was 2.5 ± 0.5. Clinical improvement was observed as early as 2-4 days after initiation of thalidomide therapy and by day 14, 5/6 patients showed clinical remission. No ENL lesions were observed in the thalidomide treated patients at day 21. As seen in The Figure, the clinical response to therapy with thalidomide occurred earlier than the response observed with the other treatment regimens.
Prednisone (30 mg/day) was used to treat six ENL patients. Two patients (patients 8 and 9) first treated with 1200 mg/day of pentoxifylline were switched to prednisone (The Table) and four patients with relapsing ENL (patients 3, 5, 12, and 13) subsequently received prednisone. Following 21 days of therapy with prednisone, 4/6 patients showed remission of ENL symptoms and lesions (The Figure).
No adverse effects were observed with the use of any of the drugs.
Plasma cytokine and cytokine receptor levels. At the time of initiation of treatment for ENL, the mean TNF-α plasma level for all patients tested was 43 ± 10 pg/ml (ranging from 6 to 159 pg/ml), the mean soluble TNF-aR level was 8.6 ± 0.8 ng/ml (ranging from 3 to 16.3 ng/ml), and the mean soluble IL-2R level was 2125 ± 273 pg/ml (ranging from 662 to 4194 pg/ml). In patients treated with either dose of pentoxifylline, there was no reduction in the plasma levels of TNF-α, soluble TNF-αR, or IL-2R. In fact, a twofold increase in TNF-α levels and a slight increase in the levels of TNF-αR and IL-2R was observed in response to pentoxifylline. This cytokine response occurred regardless of whether or not the patients showed a clinical response to pentoxifylline treatment. In contrast to the pentoxifylline associated increase in cytokines, the thalidomide treatment of ENL patients was associated with a 53% reduction in plasma TNF-α levels by 21 days postinitiation of therapy, as has been described previously (8,10'). Soluble TNF-αR was reduced by 50% and IL-2R was reduced by 43%. When ENL patients were treated with prednisone, there was an 84% decrease in the levels of TNF-α. Soluble TNF-αR was reduced by 44% and IL-2R was reduced by 37% at 21 days postinitiation of prednisone therapy.
There was no correlation between severity of ENL symptoms and the starting plasma levels of cytokine or cytokine receptors.
Since relatively low levels of TNF-α were detected in the plasma of the patients with active ENL in this study, the results suggest that TNF-α may not be the only factor in the pathogenesis of ENL. In fact, prednisone reduces TNF-α levels more efficiently than thalidomide, but the clinical response to the prednisone is somewhat slower than that observed with thalidomide treatment. Recent studies have suggested that thalidomide may also act as an immunostimulator, inducing the secretion of IL-2 by T cells (11). Whether thalidomide acts to stimulate T-cell activity in ENL patients, and the possible implications of such a stimulus on the course of ENL, remains to be determined.
- Andre L. Moreira, M.D., Ph.D.
Gilla Kaplan, Ph.D.
Laboratory of Cellular Physiology and Immunology
The Rockefeller University
1230 York Avenue
New York, New York 10021, U.S.A.
- Laarni G. Villahermosa, M.D.
Tranquilino J. Fajardo, M.D.
Rodolfo M. Abalos, M.D.
Roland V. Cellona, M.D.
Maria Victoria F. Balagon
Esterlina V. Tan
Gerald P. Walsh, Ph.D.
Leonard Wood Memorial Leprosy Research Foundation
Cebu, The Philippines
Acknowledgment. We would like to thank the nurses and attending physicians of the Leonard Wood Memorial Leprosy Research Center and the Dermatology Center, Cebu, The Philippines, for their help with this study and for the professionalism of patient care they showed. We would also like to thank Judy Adams for helping us with the preparation of The Figure, and Barbara Johnson for help with the IL-2 receptor ELISA. This study was supported by U.S. Public Heath Service grant AI-22616 and by Celgene Corporation (Warren. New Jersey. U.S.A.). All patients gave written informed consent before enrollment in the study. The protocol was approved by the ethical committee of the Leonard Wood Memorial Leprosy Research Foundation and by the IRB of The Rockefeller University, New York.
REFERENCES
1. Dezube, B. J., Pardee, A. B., Chapman, B., Beckett, L. A., Korvick, J. A., Novick, W. J., Chiurco, J., Kasdan, P., Ahlers, C. M., Ecto, L. T., Crumpacker, C. S. and The Niaid Aids Clinical Trials Group. Pentoxifylline decreases tumor necrosis factor expression and serum triglycerides in people with AIDS. J. Acquir. Immune Delic. Syndr. 6(1993)787-794.
2. Doherty, G. M., Jensen, J. C. Alexander, H. R., Buresh, C. M. and Norton, J. A. Pentoxifylline supression of tumor necrosis factor gene transcription. Surgery 7(1991)192-198.
3. Iyer, C. G. S., Languillon, J., Ramanujam, K., Tarabini-Castellani, G., De La AGUAS, J. T, Bechelli, L. M., Uemura, K., Dominguez, V. M. and Sundaresan, T. WHO coordinated shortterm double-blind trial with thalidomide in the treatment of acute lepra reactions in male lepromatous patients. Bull. WHO 45(1971) 719-732.
4. Lenz, W. A short history of thalidomide embryopathy. Teratology 38(1988)203-215.
5. McBride, W. G. Thalidomide and congenital abnormalities. Lancet 2(1961)1358.
6. Moreira, A. L., Sampaio, F. P., Zmuidzinas, A., Frindt, P., Smith, K. A. and Kaplan, G. Thalidomide exerts its inhibitory activity on tumor necrosis factor-or by enhancing mRNA degradation. J. Exp. Med. 177(1993)1675-1680.
7. Pearson, J. M. H. and VEDAGIRI, M. Treatment of moderately severe erythema nodosum leprosum with thalidomide - a double-blind controlled trail. Lepr. Rev. 40(1969) 111-116.
8. Sampaio. F. P., Kaplan. G., Miranda. A., Nery, J. A. C. Miguel, C. P., Viana. S. M. and Sarno, F. N. The influence of thalidomide on the clinical and immunologic manifestation of erythema nodosum leprosum. J. Infect. Dis. 168(1993)408-414.
9. Sampaio, E. P., Sarno. E. N., Gallily, R., COHN, Z. A. and Kaplan, G. Thalidomide selectively inhibits tumor necrosis factor-a production by stimulated human monocytes. J. Fxp. Med. 173(1991)699-703.
10. Sarno, E. N., Grau, G. E., Vieira, L. M. M. and Nery. J. A. Scrum levels of tumour necrosis factor-alpha and interleukin-1β during leprosy reactional stales. Clin. Exp. Immunol. 84(1991) 103-108.
11. Shannon F. J. and Sandoval, F. Thalidomide increses the synthesis of Il-2 in cultures of human mononuclear cells stimulated with concanavalin-A, staphylococcal enteroloxin A, and purified protein derivative. Immunopharmacology 31(1995)109-116.
12. SHESKIN, J. Thalidomide in the treatment of lepra study of methods used in clinical trials in leproreactions. Clin. Pharmacol. Ther. 6 (1965)303-306
13. WATERS, M. F. R., REES, R. J. W. and SUTHERland , I. Chemotherapeutic trials in leprosy.5. A study of methods used in clinical trials in lepro-matous leprosy. Int. J. Lepr. 35(1967)311-335.
14. ZABEL, P., Schade, F. U. and Schilaak, M. Inhibition of endogenous TNF formation by pentoxifylline. Immunobiology 187(1993)447-463.
Reprint requests to Dr. Gilla Kaplan at the above address or FAX 212-327-8875; email: kaplang@rmslab.rockefeller.edu.