- Volume 66 , Number 2
- Page: 182–9
Why Relapse Occurs in PB leprosy patients after adequate MDT Despite they are Mitsuda Reactive: lessons f rom convit's experiment on bacteria-clearing capacity of lepromin-lnduced granuloma
ABSTRACT
It is amazing how after years of scientific research and therapeutic progress many simple and basic questions about protective immunity against Mycobacterium leprae remain unanswered. Although the World Health Organization (WHO) has recommended short-term multidrug therapy (WHO/MDT) for the treatment of paucibacillary (PB) leprosy patients, f rom time to time several workers f rom different parts of the globe have reported inadequate clinical responses in a few tuberculoid and indeterminate leprosy patients following adequate WHO/MDT despite the fact that they are Mitsuda responsive. A few borderline tuberculoid patients harbor acid-fast bacilli (AFB) in their nerves for many years even though they become clinically inactive following MDT, a fact which has been ignored by many leprosy field workers. Keeping these patients in mind, we have attempted to investigate the cause of the persistence of AFB in PB cases and have looked into the question of why Mitsuda positivity in tuberculoid and indeterminate leprosy patients, as well as in healthy contacts, is not invariably a guarantee for protectivity against the leprosy bacilli. We have: a) analyzed the histological features of lepromin-induced granulomas, b) studied the bacteria-clearing capacity of the macrophages within such granulomas, and c) studied the in vitro leukocyte migration inhibition factor released by the blood leukocytes of these subjects when M. leprae sonicates have been used as an elicitor. The results of these three tests in the three groups of subjects have been compared and led us to conclude that the bacteria-clearing capacity of the macrophages within lepromin-induced granuloma (positive CCB test) may be taken as an indicator of the capability of elimination of leprosy bacilli and protective immunity against the disease. This important macrophage function is not invariably present in all tuberculoid and indeterminate leprosy patients or in all contacts even though they are Mitsuda responsive and are able to show a positive leukocyte migration inhibition (LMI) test. It is likely but not certain that this deficit of the macrophage is genetically predetermined and persists after completion of short-term WHO/MDT. Thus, after discontinuation of treatment slow-growing, persisting M. leprae multiply within macrophages leading to relapse.RÉSUMÉ
Il est surprenant de constater qu'après tant d'années de recherche scientifique et de progrès thérapeutiques, autant de questions simples et de base demeurent sans réponse au sujet de l'immunité protectrice contre Mycobacterium leprae. Bien que l'Organisation mondiale de la Santé (OMS) ait recommendé une polychimiothérapie (PCT/OMS) à court terme pour le traitement des patients lépreux paucibaeillaires (PB), il n'en reste pas moins que plusieurs personnes à travers le monde ont rapporté épisodiquement des réponses cliniques inadéquates chez quelques patients tuberculoïdes et indéterminés, consécutivement á une PCT /OMS adéquate, malgré le fait que ces patients réagissent au test de Mitsuda. Quelques patients borderlines tuberculoïdes présentent pendant des années des bacilles acido-alcolo-résistant (AAR), même s'ils deviennent cliniquement inactifs, un lait qui a été largement ignoré par de nombreux léprologistes de terrain. Avec l'histoire de ces patients en tête, nous avons tenté d'explorer la cause de persistance de ces bacilles AAR chez les patients PB et avons tenté de savoir pourquoi une positivité au test de Mitsuda chez les patients tuberculoïdes ou indéterminés, ainsi que les personnescontactes non affectées, n'est pas nécessairement une garantie de protection contre les bacilles de la lèpre. Nous avons: a) analysé les caractères histologiques des granulomes induits par l'injection de Lépromine. b) étudié la capacité d'élimination bactérienne des macrophages provenant de tels granulomes. c) étudié in vitro le factor d'inhibition de migration des leucocytes sécrété par les leucocytes sanguins de ces sujets lorsque des sonicates de M. Leprae furent utilisés comme stimulateurs. Les résultats de ces 3 tests chez les 3 groupes de sujets ont été comparés, ce qui nous a permis de conclure que le test de capacité à éliminer les bactéries des macrophages provenant de granulomes induits par le lépromine peut être considéré comme un indicateur delà capacité d'élimination du bacille de la lèpre et de l'immunité protectrice contre la maladie. Cette importante fonction du macrophage n'est pas invariablement présent chez, tous les patients tuberculoïdes ou indéterminés ou chez toutes les personnes contactes même s'ils sont tous positifs au test de Mitsuda et capables démontrer un test d'inhibition de migration leucocytaire (IML) positif. Il est probable, mais pas certain, que ce délicit des macrophages est génétiquement prédéterminé et persiste après l'arrêt de la PCT/OMS à court terme. Ainsi, après l'interruption du traitement, des M. Ieprae persistantes, à croissance lente, se multiplient à l'intérieur des macrophages, aboutissant à des rechutes.RESUMEN
Es sorprendente como después de tantos años de investigación científica y de progreso terapéutico todavía queden sin contestar muchas preguntas simples y básicas sobre la inmunidad protectora contra Mycobacterium leprae. Aunque la Organización Mundial de la Salud (OMS) ha recomendado la poliquimioterapia de corta duración apra el tratamiento de la lepra paucibacilar (PB), todavía, de tiempo en tiempo, algunos investigadores de dieferentcs partes del mundo reportan respuestas clínicas inadecuadas en algunos pacientes con lepra tuberculoide c indeterminada, a pesar de qeu han recibido el tratamiento (PQT) apropiado y de su respuesta Mitsuda positiva. Algunos pacientes con lepra tuberculoid subpolar mantienen bacilos ácido-resistentes (AFB) en sus nervios durante muchos años aunque se hayan tornado clínicamente inactivos después de la PQT, un hecho que ha sido ignorado por muchos investigadores de la lepra. Manteniendo estos cases en la mente, hemos intentado investigar la causa de la permanencia de AFB en los casos PB y hemos enfocado la atención a la pregunta de por qué la positividad a Mitsuda en los pacientes tuberculoides, en los pacientes indeterminados y en los contactos sanos, no es invariablemente una garantía de protección contra el bacilo d e la lepra. Para esto hemos: a) analizado las características histológicas de los granulomas inducidos con lepromina. b) estudiado la capacidad de los macrófagos de los granulomas para depurar bacterias, y c) estudiado la liberación del factor inhibidor de la migración de leucocitos por los leucocitos circulantes de estos sujetos cuando se estimulan in vitro con sonicados de M. leprae. Los resultados comparitivos de estas 3 pruebas en los 3 grupos de individuos han conducido a concluir que la capacidad depuradora de bacterias de los macrófagos de los granulomas inducidos con lepromina puede tomarse como un indicador de la capacidad de eliminación del bacilo de-la lepra y de la inmunidad protectora contra la enfermedad, lista importante función macrofágica no está invariablemente presente en todos los paciente tuberculoides e indeterminados, ni en todos los contactos sanos aunque sean Mitsuda-positivos y capaces demostrar un prueba de inhibición de leucocitos positiva. Es probable que el délicit de los macrófagos esté predeterminado genéticamente y que persista aun después de completarse el tratamiento (PQT) de corta duración sugerido por la OMS. Así, al descontinuar el tratamiento, los bacilos de la lepra persistentes pueden replicarse lentamente dentro de los macrófagos dando origen a las recaídas.It is generally agreed that at the lepromatous pole of the leprosy spectrum there exists anergy toward Mycobacterium leprae and an absence of protective immunity against the parasite, resulting in unrestricted growth of acid-fast bacilli (AFB) within Virchowian macrophages and the development of extensive disease. On the other hand, at the tuberculoid pole there is strong Mitsuda positivity but incomplete' protective immunity against M. leprae, permitting its restricted multiplication and development of limited disease. Although tuberculoid (TT) and borderline tuberculoid (BT) leprosy patients harbor few AFB, one million or less, and can mount a significant granulomatous delayed-type hypersensitivity (DTH) reaction against the leprosy bacilli, the tuberculoid granuloma especially within the nerve does not resolve in some patients even after the recommended short-term multidrug therapy (MDT) (8). Often they stand a chance of relapsing after discontinuation of MDT, perhaps due to persisting slow-growing M. leprae. Also they develop nerve damage induced either by the remnant M. leprae antigens, which the host fails to eliminate (13), or by the host's own antigenic determinants similar to those of M. leprae (17).
Indeterminate leprosy is immunologically unstable. Some patients remain unchanged for years, even without treatment, or undergo spontaneous regression, while a small fraction, despite being Mitsuda-reactive, change their polarity mostly toward the tuberculoid pole. Dharmendra and Chatterjee (6) in a retrospective study have shown that out of 524 lepromin-positive healthy contacts, 17 (3.2%) developed tuberculoid leprosy. This indicated that their cell-mediated immunity (CMI) was not competent enough to eliminate all leprosy bacilli, although they were able to form a DTH granuloma at the lepromin injection sites (6). Kar and associates (9) reported that 3 (6%) out of 50 indeterminate leprosy patients remained clinically active even at the end of 1 year's treatment with MDT. Kaur and her coworkers (10) reported inadequate clinical response in 4.3% and 25% of her tuberculoid and indeterminate leprosy patients, respectively, following MDT.
Why some of these patients fail to eliminate all leprosy bacilli although they are Mitsuda positive and possess adequate CMI against M. leprae is still not clearly understood. It appears that protective immunity is the most important and under-investigated aspect of the disease.
Keeping this in mind, in this study we attempted to investigate the nature of protective immunity in leprosy. We induced DTH granulomas in Mitsuda-positive tuberculoid and indeterminate leprosy patients as well as in Mitsuda-positive healthy contacts by intradermal injection of 6.4 x 107 heat-killed M. leprae prepared from human leproma (3) and studied any functional defect of the lymphocytes and macrophages that had infiltrated into the granulomas from the vascular bed.
MATERIALS AND METHODS
Human materials
The study included three groups of human subjects. Group I included 52 histologically proved, tuberculoid leprosy patients. They were all bacteriologically negative by slit-skin-smear tests, and all were Mitsuda positive. The lepromin test was performed by intradermal injection of 0.1 ml standard lepromin containing 1.6 x 108 heat-killed M. leprae (human) per ml; 3 weeks thereafter the Mitsuda reaction was recorded (7). Short-term MDT was given to all patients.
Group II included 50 Mitsuda-positive, histologically proved, indeterminate leprosy patients.
Group III consisted of 25 Mitsuda-positive healthy contacts (5 to 40 years old). They were selected from families having lepromatous (LL) and borderline lepromatous (BL) patients.
Laboratory tests
Three types of laboratory tests were performed.
Histological study of lepromin (megadose)-induced dermal granuloma. Briefly, 6.4 x 107 heat-killed M. leprae (human) (four times dose of a standard lepromin test) were injected intradermally into each subject. Six weeks thereafter biopsies were taken from the inoculation sites, and histological sections were made and stained with hematoxylin and eosin (H&E) to study the histomorphology of the granuloma formed therein (5).
Capacity of clearing bacteria (CCB) test. The CCB test was done by studying the histological sections of the lepromin (megadose)-induced granuloma (described above) after Fite-Faraco staining for AFB. The results were recorded according to the method described by Convit and associates (5). Clearance of bacteria indicated a positive test.
Leukocyte migration inhibition (LMI) test. Leukocyte-rich plasma samples were obtained from each subject; cell suspensions containing 4 X 106 cells per ml of minimal essential medium (MEM) were used to fill the capillary tubes; these were then incubated in migration chambers filled with MEM and 10% fetal calf serum with or without 1 x 107 AY. leprae sonicates. Areas of migration were measured and migration indices were calculated (3). A migration index below 80 indicates LMI positivity; above 80, LMI negativity.
The results of these three tests in the patients and contacts were compared.
RESULTS
The Table shows the results of the three tests performed for the tuberculoid and indeterminate leprosy patients as well as for the contacts. The histological characteristics of a typical lepromin granuloma are: the presence of Langhans' giant cells, epithelioid cells, extensive lymphoid-cell infiltrates, and circumscribed tuberculoid structure. An atypical granuloma is characterized by the absence of macrophage differentiation, limited or no development of epithelioid cells and scarce lymphoid infiltration as well as the absence of giant cells (5).
All 52 TT leprosy patients were Mitsuda positive, but 6 patients had an atypical granuloma and the remaining 46 patients showed a typical granuloma. Fourteen TT patients, including the above 6 patients showing atypical granuloma and another 8 patients showing a typical granuloma, failed to clear dead M. leprae (solid or fragmented) from the lepromin-induced granuloma (negative CCB test). Twelve patients had a negative LMI test and the remaining 40 patients showed a positive LMI test. Again, of the 40 LMI-positive TT leprosy patients, 38 patients could show CCB positivity, indicating a close correlationship between the LMI and CCB tests. Curiously, the remaining two Mitsuda-reactive TT patients were LMI positive but CCB negative. Amazingly, of the 46 Mitsuda-positive TT leprosy patients showing typical granuloma at the lepromin injection sites, eight were CCB negative with Langhans' giant cells within granuloma showing intracellular AFB (The Figure). Essentially all individuals were Mitsuda positive, while only a portion showed CCB and LMI reactivities. Mitsuda reactivity could be a more sensitive detector of CMI but Mitsuda positivity is not always associated with CCB and LMI positivities.
The results of the three tests in the 50 Mitsuda-positive indeterminate leprosy patients were similar (The Table). Of these 50 patients, 49 (98%) showed a typical granuloma and only one showed atypical granuloma at the lepromin inoculation sites. Forty-nine patients had typical and one had atypical granulomas; 28 (56%) patients showed CCB positivity and 22 (44%) CCB negativity (the latter showing mostly fragmented AFB even within Langhans' giant cells).
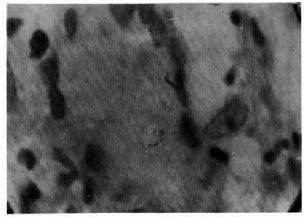
The figure. Langhans' giant cell with intracellular acid-fast bacilli within a "typical" granuloma induced by intradermal injection of a large dose of lepromin in a TT case (negative CCB test) (Fite-Faraco x1000).
An in-vitro test was positive in 38 (76%) out of the 50 indeterminate leprosy patients but a CCB test was positive in only 28 (56%) cases which, again, indicated that all LMI-positive patients were Mitsuda positive but not CCB positive. However, all CCB-positive cases invariably showed Mitsuda and LMI positivities.
The results in the 25 Mitsuda-positive healthy contacts were also similar (The Table). All 25 Mitsuda-positive contacts showed a typical lepromin granuloma but, unlike the tuberculoid and indeterminate patients, none showed an atypical granuloma. Nevertheless, two such typical giant-cell granulomas had AFB. Of these two granulomas, one showed AFB within the Langhans' giant cells. Out of 25 contacts, 23 showed both CCB and LMI positivities, again indicating a close correlation between the results of these two tests. Importantly, the bacteria-clearing competency of the lepromin-driven granuloma and lymphokine release by specific T cells following challenge with M. leprae antigen in vitro were impaired even in some Mitsuda-positive healthy contacts. These data indicated that a few Mitsuda-positive PB leprosy patients and contacts showing typical giant-cell granuloma induced by lepromin might not clear a few, mostly fragmented, AFB from the inoculation sites. Presumably they were not resistant against M. leprae.
DISCUSSION
The study group composed of 127 subjects [52 tuberculoid (TT) patients, 50 indeterminate patients and 25 healthy contacts] were all Mitsuda positive. They were subjected to histopathological examination of lepromin (negative) injected for granuloma function, and CCB and LMI tests.
Convit, et al. (5) developed an in-vivo (CCB) test to find out the competency of the macrophage to eliminate dead AFB from the granulomas induced by intradermal injection of a megadose of lepromin in patients suffering from various types of leprosy. They found that Mitsuda-unresponsive LL patients formed an atypical and unprofessional granuloma, wherein macrophages not only failed to differentiate into epithelioid and giant cells, but also showed persistence of intracellular AFB. On the other hand, a similar test performed in Mitsuda-positive tuberculoid leprosy patients led to the formation of a typical and professional DTH granuloma, wherein most macrophages underwent differentiation into epithelioid cells and a few multinucleated Langhans' giant cells, capable of eliminating intracellular AFB (19). It is well known that the presence of Langhans' giant cells in a lepromin granuloma is variable and does not relate to the degree of CMI. Langhans' giant cells are often absent in polar tuberculoid (TTp) cases, conspicuous in subpolar (TTs) and small in size in borderline (BT) leprosy cases (18). Curiously, we found both typical and atypical granulomas at the lepromin injection sites of our Mitsuda-positive TT and indeterminate cases.
The results of the CCB tests in our 127 Mitsuda-positive patients and contacts showed that 89 (70%) could clear AFB from the lepromin-induced granuloma (The Table) and obeyed Convit's rule, while the rest did not (The Table). In their original paper, Convit, et al. (5) considered the persistence of a few bacilli or bacillary debris in some Mitsuda-positive patients as CCB positive. However, in the present study to avoid subjectivity persistence of fragmented acid-fast materials, although small in number (The Figure), was taken as CCB negative.
Also six (11 %) TT patients of the present study, although Mitsuda-positive, formed an atypical granuloma containing AFB (CCB negative).
Furthermore, even giant cells within a typical lepromin granuloma showed AFB in 31 Mitsuda-reactive TT and indeterminate patients as well as contacts (The Table). They were CCB negative, and of them 26 cases showed negative LMI tests and the remaining five cases were LMI positive, indicating thereby that CCB and LMI negativities may not give parallel results in a few cases (The Table). M. leprae and their debris persisting in these Mitsuda-positive, CCB-negative cases even after completion of short-term MDT might mount a cell-mediated immune response, eventually leading to a type 1 reaction and nerve damage (13).
These paradoxical findings, i.e., few Mitsuda-positive TT and indeterminate leprosy patients forming typical and atypical granulomas at the lepromin injection sites and showing CCB negativity and variable LMI reactivities, are of interest. For the assessment of resistance of an individual against M. leprae CCB positivity, an indicator of protective immunity, outstrips the other two parameters, i.e., Mitsuda reactivity, an indicator of granulomatous hypersensitivity, and LMI positivity, a pointer of specific T-cell responses against M. leprae in vitro (7).
It has now been established that intracellular killing of mycobacteria and their subsequent elimination involve a multistep process; i.e., uptake and processing of AFB by macrophages, presentation of the processed mycobacterial antigen onto the Th 1 subset of CD4 cells through MHC molecules followed by the production of paracrine gamma interferon and autocrine tumor necrosis factor-alpha. These stimulate bacteria-laden macrophages to differentiate into epithelioid cells and giant cells as well as lymphocyte infiltration with eventful killing and elimination of intracellular bacteria (2, 16). The result of our LMI tests showed that M. feprae-specific T cells in 101 (80%) of 127 of our Mitsuda-positive patients and contacts were able to release the migration inhibitory factor when they were challenged with M. leprae sonicates in vitro (LMI positivity) (The Table). However, 38 (37%) out of 101 LMI-positive cases were CCB negative, suggesting impaired macrophage function despite having an intact T-cell response. These findings indicate that either the LMI test is less sensitive than Mitsuda reactivity or that LMI positivity is not an indicator of the resistance of macrophages against M. leprae (1). However, both LMI and CCB positivities are interdependent and need T cell help and macrophage activation. Also, it was reported earlier that thymus-derived lymphocytes obtained from tuberculoid granulomas exhibited exaggerated activities as evidenced by a high incorporation of 3H-thymidine and l4G-leucine (15), while the bone-marrow-derived macrophages therein were armed with HLA-DR (la) antigens after being activated by specific T cells (14). Thus, clearing of dead M. leprae from the lepromin-induced granuloma demands the T-cell-mediated activation of macrophages and the subsequent differentiation into epithelioid and Langhans' giant cells. Atypical granulomas without any epithelioid or giant cells were inert and could not eliminate AFB (CCB test negative), even Langhans' giant cells within typical granulomas formed at the lepromin injection sites of 31 patients (The Table) contained AFB, showing thereby that Langhans' giant cells in these patients have a slower digestive process than in others and perhaps were less active.
We are tempted to postulate that differentiation of macrophages into multinucleated Langhans' giant cells and their ability to digest and to eliminate intracellular mycobacteria are two distinct events: the first certainly needs T cell help, the second needs intact macrophage function which is perhaps genetically predetermined and associated with the Ir gene. The Ir gene is the human equivalent to the beg gene in mice which is responsible for innate resistance or susceptibility to mycobacterial infection (12). The Ir gene controls the MHC class II antigen expression, affects respiratory burst, and kills intracellular organisms (16). An experimental proof is available. A strain of mice susceptible to M. lepraeniuriuin when challenged with different doses of the pathogen developed the disease at a certain dose but showed strong skin DTH reaction against the same pathogen (11). Thus, the observed defective bacteria-clearing capacity of the macrophages within the tuberculoid granuloma of a patient is perhaps genetically predetermined and tends to persist following adequate MDT. It is now known that the bcgr allele confers resistance and is dominant over bcg\ Also beg' macrophages are superior to bcgs macrophages in the expression of surface markers (renamed as natural resistance-associated macrophage protein, NRAMP) and are associated with the activation of toxic nitrogen and oxygen radicals (2, 20, 21) . This inability of macrophages to differentiate into Langhans' giant cells within the lepromin-driven granuloma and their inability to clear intracellular AFB in some of our Mitsuda-positive patients and contacts is perhaps due to the fact that gamma interferon released by Thl cells fails to activate the human equivalent of the beg" macrophages and to kill and release intracellular mycobacteria (2, 10, 18). Alternatively, defective autocrine release of tumor necrosis factor-alpha by such macrophages fails to cause them to mature into typical Langhans' giant cells (2). Certainly this needs experimental proof.
We would like to highlight that even today we do not understand the meaning of the Mitsuda reaction, i.e., lepromin-induced DTH granuloma. It is believed that naturally induced DTH reactivity against M. leprae, which is life long, strongly correlates with protection (2). On the contrary, a vaccine-induced Mitsuda positivity wanes over the course of time (12). This difference in the behavior of Mitsuda positivity, natural or induced, is perhaps of genetic origin and needs to be addressed in a subsequent study. However, the results of our present study have shown that lepromin-induced granuloma, typical or atypical, may or may not eliminate AFB from the granuloma and, thus, have put forward evidence that CCB positivity is a better indicator of protective immunity than Mitsuda positivity. These two tests could be evaluated in PB patients by doing a long-term follow up after therapy to determine if relapses were more frequent in the CCB-negative cases.
Very recently, Job and his coworkers (8) have reported persistence of AFB in the endoneurium of the tibial nerve of a BT leprosy patient even 21 years after adequate antileprosy therapy. They have pointed out that BT leprosy patients should be considered as a generalized disease and should be given a longer duration of currently available antileprosy therapy. Such cases, if CCB-negative, will not respond to short-term WHO/MDT treatment and will not be able to eliminate all AFB. We, therefore, advocate that Mitsuda-positive but CCB-negative tuberculoid and indeterminate leprosy patients be administered low-dose Convit vaccine plus MDT for quick clearance of bacteria, as done in Mitsuda-negative multibacillary and paucibacillary leprosy patients (4, 11).
REFERENCES
1. BJUNE, G., BARNETSON, R. ST. C, RIDLEY, J. S. and KRONWALL, G. Lymphocyte transformation test in leprosy: correlation of response with inflammation of lesions. Clin. Exp. Immunol. 25(1976)80-94.
2. CHAUDHURI, K. The immunology of leprosy: unveiling an enigma. (Editorial) Int. J. Lepr. 63(1995)430-447.
3. CHAUDHURY, S., HAJRA, S. K., SAHA, B., MASUNDER, B., BISWAS, P. C, CHATTAPADHYA, D. and S AHA, K. An eight-year held trial on antileprosy vaccines among high-risk household contacts in the Calcutta metropolis. Int. J. Lepr. 62(1994)369-394.
4. CHAUDHURY, S., HAJRA, S. K., SAHA, B. and SAHA, K. Management of lepromin negative borderline leprosy patients with low dose Convit vaccine as an adjunct to multidrug therapy (a six-year follow up study at Calcutta). Int. J. Lepr. 65(1997)56-62.
5. CONVIT, J., AVILA, J. L., GOIHMAN-YAHR, M. and PINARDI, M. E. A test for determination of competency in clearing bacilli in leprosy patients. Bull. WHO 46(1972)821-826.
6. DHARMENDRA and CHATTERJEE, K. R. Prognostic-value of the lepromin test in contacts of leprosy cases. Lepr. India 27(1955)149-152.
7. HARBOE, M. The immunology of leprosy. In: leprosy. Hastings, R. C, ed. Edinburgh: Churchill Livingstone, 1985, pp. 53-87.
8. JOB, C. K., BASKARAN, B., JAYAKUMAR, J. and ASCHHOFF, M. Pathologic changes in a tibial nerve with surviving M. leprae in a healed tuberculoid leprosy patient. Int. J. Lepr. 65(1997)90-94.
9. KAR, P. K., JHA. P. K. and SNEHI, P. S. Indeterminate leprosy: a therapeutic evaluation. Indian J. Lepr. 64(1992)163-167.
10. KAUR, S., SHARMA, V. K., BASAK, P. and KAUR, I. Paueibacillary multi-drug therapy in leprosy: 7½ years experience. Indian J. Lepr. 64(1992)153-161.
11. LOVIK, M. and GLOSS, O. Repeated delayed-type hypersensitivity reaction against Mycobacterium lepromurium antigens at the infection site do not affect multiplication in CSH mice. Infect. Immun. 36(1982)768-774.
12. MAJUNDER, V., MUKHERJEE, A., HAJRA, S. K., SAHA, B. and SAHA. K. Immunotherapy of far-advanced patients of lepromatous leprosy with low dose Convit vaccine with multidrug therapy (a Calcutta trial). Int. J. Lepr. 64(1996)26-36.
13. NAAFS, B. Features of relapse in paucibacillary leprosy after multi-drug therapy. Indian J. Lepr. 67(1995)61-67.
14. NARAYANAN. R. B. Immunopathology of leprosy granulomas; current status: a review. Lepr. Rev. 59(1988)75-82.
15. NARAYANAN, R. B. and GlRDHAR, B. K. In-vitro studies on dermal leprosy granulomas: assessment of division and protein synthesis of cells. Acta Leprol. 7(1989)13-17.
16. OTTENHOFF, TOM, H. M. Immunology of leprosy: lesson from and for leprosy (Editorial). Int. J. Lepr. 62(1994)108-121.
17. RAMBUKKANA, A., DAS. P. K., KROIG, S., YONG, S., SEPOOLE, I. C. and BOS, J. D. Mycobacterial 65.000 MW heat-shock protein shares a carboxyterminal epitope with human epidermal cyteberatin-1/2. Immunology 77(1992)267-276.
18. RIDLEY, D. S. Pathogenesis of Leprosy and Related Diseases. London: Butterworth & Co. Ltd., 198S, pp. 157-159.
19. ROSEN, F. S. and GEHN, R. S. Chronic granulomatous disease. In: Case Studies in Immunology; A Clinical Comparison. London: Current Biology Limited. 1996, pp. 67-72.
20. SCHURR. E., MALO. D., RADZIOCH, D., BUSCHMAN, E. MORGAN, K., GROS, P. and SKAMENE, K. Genetic control of innate resistance to mycobacterial infections. Inmmnoparasitol. Today 12(1991)A42-A45.
21. SCHURR, E., MORGAN, K., GROS, P. and SKAMENE, E Genetics of leprosy. Am. J. Trop. Med. Hyg. 44(1991)4-11.
1. S. Chaudhuri, M.B.B.S., D.C.P., Ph.D., Professor of Leprology (deceased).
2. S. K. Hajra, M.B.B.S., D.C.P., D.T.M. & H., Ph.D., Reader of Leprology (retired).
3. A. Mukherjee, M.D., Ph.D., D.C.P., Professor of Pathology and Director (retired).
4. B. Saha, M.D., D.T.M.&H., Assistant Professor., Demonstrator, Department of Leprosy, School of Tropical Medicine, Calcutta, India.
5. B. Mazumder, M.B.B.S., D.D.V., Demonstrator, Department of Leprosy, School of Tropical Medicine, Calcutta, India.
6. D. Chattapadhya, M.D., Joint Director, National Institute of Communicable Diseases, Delhi, India.
7. K. Saha, M.Sc, M.B.B.S., Ph.D. (U.S.A.), Professor of Immunology (retired), Delhi University, Delhi, India.
Reprint requests to Dr. K. Saha, 45A Sova Bazar Street, Calcutta 700 005, India.
Received for publication on 2 July 1997.
Accepted for publication in revised form on 22 April 1998.