- Volume 66 , Number 2
- Page: 208–13
Two-dimensional electrophoretic analysis of humoral responses to culture filtrate of Mycobacterium bovis BCG in patients with leprosy and tuberculosis
ABSTRACT
Sera f rom 3 lepromatous (LL), 3 borderline lepromatous (BL), 3 mid-borderline (BB), 3 borderline tuberculoid (BT), 2 tuberculoid (TT) and 4 tuberculosis (TB) patients and 3 healthy individuals were examined for their reactivities against the proteins in the culture filtrate of BCG separated by two-dimensional electrophoresis (2-DE). The sera were obtained f rom patients who were untreated. Sera f rom LL and BL patients reacted strongly with the antigen 85 (Ag85) complex and MPB51. Sera f rom LL and BL patients also weakly reacted with the newly identified 29-, 24- and 23-kDa spots. Sera f rom the other patients reacted similarly, but the levels of reaction were remarkably lower than those f rom LL and BL patients. Mycobacterium leprae antigens that are analogous to BCG Ag85 and MPB51 are suggested as the main targets for the humoral immunity of untreated patients. The reactivities of sera with newly identified antigens may provide the potential for predicting the severity and prognosis of diseases.RÉSUMÉ
Les serums collectés à partir de patients hanséniens incluant 3 lépromateux (LL). 3 lépromateux borderlines (BL). 3 borderlines (BB). 2 tubereuloïdes (TT). ainsi que 4 patients tuberculeux (TB) et 3 individus en bonne santé lurent analysés pour leur réactivité contre les protéines obtenues à partir de filtrats de cultures de BCG. protéines séparées par électrophrèse à 2 dimensions (2-DE). Les sérums furent obtenus à partir de patients non traités. Les sérums des patients LL et BL ont fortement réagis contre le complexe antigénique 85 (Ag 85) et MPB51. Les sérums des patients LL et BL ont aussi faiblement réagis avec des spots nouvellement identifiés de 29. 24 et 23 kilodaltons (kDa). Les sérums des autres patients ont réagis de façon similaire, avec toutefois des niveaux de réaction très nettement inférieurs à ceux observés chez les patients LL et BL. Il est supposé que les antigènes de M. leprae qui sont analogues à ceux des antigènes Ag85 et MPB51 du BCG sont les cibles principales de l'immunité à médiation humorale des patients non traités. La présence d'une réaction de ces sérums avec des antigènes nouvellement identifiés pourrait potentiellement permettre tic prédire la sévérité et le pronostic de maladies.RESUMEN
Se probaron los sueros de 3 pacientes lepromatosos (LL), 3 lepromatosos subpolares (BL), 3 pacientes intermedios (BB), 3 Uiberculoides subpolares (BT). 2 tuberculoides (TT), 4 pacientes con tuberculosis (TB), y 3 individuos sanos, para establecer su reactilidad contra las proteínas secretadas por BCG fraccionadas por eletroforesis bidimensional (2-DE). Todos los individuos fueron cases no tratados. Los sueros de los pacientes LL y BL reaccionaron fuertemente con el complejo antigénico 85 (Ag 85) y con MPB51. Los sueros de los pacientes LL y BL también reaccionaron débilmente con moléculas novelas de 29-. 24- y 23 kDa. Los sueros de los otros pacientes reaccionaron de manera similar pero los niveles de reacción fueron marcadamente menores que los de los pacientes LL y BL. Se sugiere que los antígenos de Mycobacterium leprae que son análogos a los antígenos Ag 85 y MPB5I de BCG sean los blancos principales de la inmunidad humoral en los pacientes no tratados. Las reactividades de los sueros con los nuevos antígenos identificados podrían ser de utilidad potencial para predecir la severidad y el pronóstico de la enfermedad.Leprosy still remains a major chronic infectious disease affecting more than 1,260,000 patients. There are two polar types of the diseases. One is lepromatous leprosy (LL), which represents no specific cellular immune response to Mycobacterium leprae and has a large number of bacilli within phagocytes in the macrophage-rich granuloma. The other is tuberculoid leprosy (TT), which is characterized by a vigorous cellular immune response and can result in irreversible nerve destruction in a high proportion of cases. Precise early diagnosis is important for the initiation of efficient chemotherapy. A variety of techniques to detect an infection with M^ leprae have been offered. In prognosis and treatment, there are differences between the LL and TT types of the disease. Many studies on the diagnosis of leprosy also have been reported (4, 12).
The antigen 85 (Ag85) complex, Ag85-A = MPB44 (9), Ag85-B = MPB59 = α antigen (6) and Ag85-C = MPB45 (9), is secreted by mycobacteria. These antigens are presented both on the cell surface and in the cytosol of M. leprae within the infected lesions of LL patients (13). The humoral responses to Ag85 complex have been reported by different groups (5, 17). However, so far no comparisons of the reactions with whole secreted proteins have been presented.
The genes for the culture filtrate (CF) proteins, a antigen (6), MPB51 (11), MPB64 (18), MPB70 (15), MPB83 (7), and MPB57 (19) were first cloned by us from M. bovis bacillus Calmette-Guerin (BCG). On the basis of this background, comparative analyses were carried out on humoral responses of patients against whole CF proteins in the hope that these proteins might contain antigens useful in predicting the prognosis of the disease. Two-dimensional electrophoresis (2-DE) has great resolving power to separate individual proteins. The CF of BCG was analyzed by 2-DE. Ag85 complex and MPB51 were immunodominant antigens as previously reported (12). In addition, we identified three new antigens that were recognized by leprosy sera.
MATERIALS AND METHODS
Serum. Patients were classified clinically and histopathologically. Serum samples were collected from 3 LL, 3 borderline lepromatous (BL), 3 mid-borderline (BB), 3 borderline tuberculoid (BT), 2 TT and 4 tuberculosis (TB) patients (The Table). All patients were studied before chemotherapy. The healthy control sera were collected from three persons who had no prior history of exposure to leprosy.
CF. BCG substrain Tokyo was used in this study. It was cultured on the surface of Sauton liquid medium for 3 weeks. The culture was passed through a membrane filter with a pore size of 0.45 µm to remove BCG cells. The proteins in the filtrate were concentrated by solid ammonium sulfate at 80% saturation, as described previously (8), and the concentrate was dialyzed against TMNSH buffer (10 mM Tris-HCl buffer, pH 7.8, 10 mM Mg acetate, 60 mM NH4CI and 6 mM 2-mercaptoethanol).
2-DE. The proteins in the CF were analyzed by 2-DE according to the method of O'Farrell (10) with a few modifications as described previously (9). In each 2-DE gel, 20 µg of CF protein was loaded. The first dimension was isoelectric focusing in 8 M urea with a 2% ampholyte mixture (Bio-Lyte; Bio-Rad mixture of 3/10:3/5:5/7 = 4:1:1) (Bio-Rad Laboratories, Hercules, California, U.S.A.). The second dimension was sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) with a 12.5% polyacrylamide gel. The gels were processed for Western blotting or proteins were visualized by silver staining.
Immunoblotting. Immunoblotting was carried out according to the method of Tow-bin, et al. (16). The blots were probed with 1:100 diluted serum antibodies, and detected with 1:1000 diluted peroxidase-conjugated rabbit antihuman immunoglobulin G (Dako A/S, Glostrup, Denmark).
RESULTS
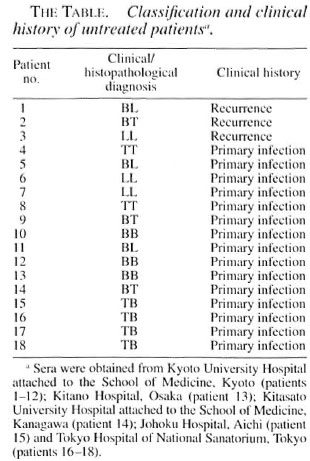
2-DE. 2-DE of the CF derived from BCG is presented in Figure 1. The silver-stained gel showed the well-separated proteins. For example, Ag85-A, α antigen, AgS5-C, MPB51, MPB64, MPB70, the 65kDa protein (groEL/hsp60 analog) (14), MPB57 (groES/hsplO analog), and BCG 45/47-kDa complex (9) were defined as clear spots.
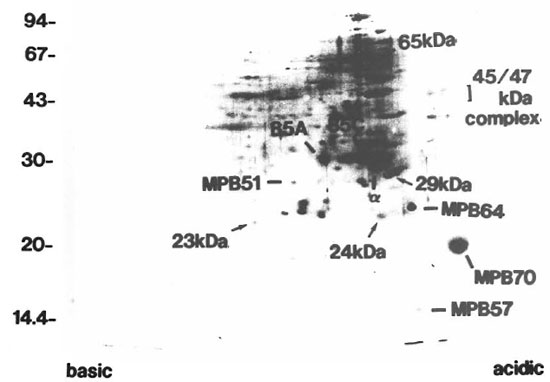
Fig. 1. 2-DE of CF. proteins derived from BCG. The gel was visualized by silver staining. Apparent sizes ofmolecular weight standards are indicated in kDa along the left side. 85A = Ag85-A; α = antigen; 85C = Ag85-C; 65 kDa = 65-kDa protein (groEL analog); MPB = major protein secreted from BCG.
Humoral responses of LL and BL patients to CF proteins. Represented in Figure 2A, all sera showed the strongest humoral reactions to Ag85 complex and MPB51 among the CF proteins. A majority of the sera reacted to BCG 45/47-kDa complex (5/6 = 83%). Only a smaller number of sera recognized the MPB57 and 65-kDa protein spots (MPB57: 1/6 = 16.7%, 65-kDa: 3/6 = 50%). These responses were weaker than those to the Ag85 complex and BCG 45/47-kDa complex. There were also three small seroreactive spots, the 29-, 24- and 23-kDa spots, that had not as yet been characterized (arrows in Fig. 2). All sera reacted weakly to the 29- and 24-kDa spots. Concerning the 23-kDa spot, half of the sera gave a positive reaction.
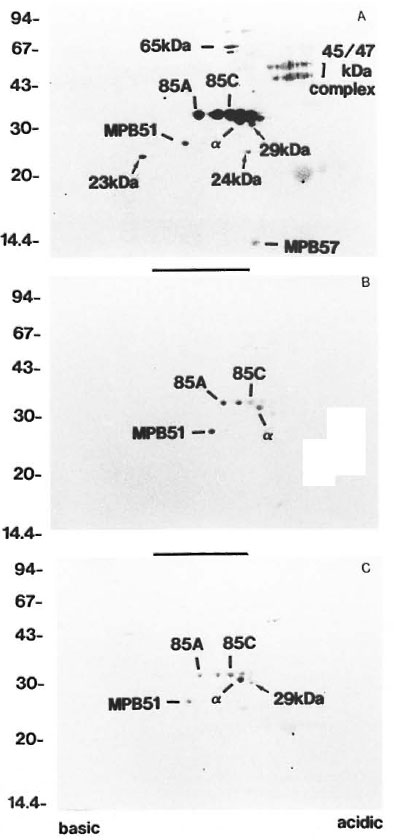
Fig. 2. Patterns of antibody responses to CI: proteins separated by 2-DE of three individual patients: A =LL,B = TT and C = TB.  = spots with molecular weights of 29-, 24-, and 23-kDa. Apparent sizes of molecularweight standards are indicated in kDa along the left side; other spots are as indicated in Figure 1.
= spots with molecular weights of 29-, 24-, and 23-kDa. Apparent sizes of molecularweight standards are indicated in kDa along the left side; other spots are as indicated in Figure 1.
Humoral responses of BB, BT and TT patients to CF proteins. Proteins were recognized relatively less intensely or not at all when probed with BB, BT and TT sera (Fig. 2B). All of these sera showed weak reactions against Ag85 complex and MPB51, but only 1 out of 3 BB and 1 out of 3 BT sera showed strong reactions. Other spots were recognized with only a few sera (MPB57: 2/8 = 25% ; 65-kDa: 2/8 = 25%; BCG 45/47-kDa complex: 1/8= 12.5%; 29kDa: 3/8 = 37.5%; 24-kDa: 2/8 = 25%; 23-kDa: 1/8 = 12.5%). There were no spots that were recognized by only the BT and TT patients.
Humoral responses of TB patients to CF proteins. TB patients showed reactions similar to the TT and BT patients. Ag85 complex, MPB51 and 29-kDa spots were weakly recognized by these sera (Fig. 2C). Other spots were recognized with some sera (65-kDa: 3/4 = 75%; 24-kDa: 1/4 = 25%; 23-kDa: 1/4 = 25%). MPB57 showed no reaction with any sera. The humoral responses of healthy control sera showed no reactions to any of the CF proteins (data not shown).
DISCUSSION
One of the main limitations on the successful control of leprosy as a public health problem is the lack of methods for its early diagnosis in subclinical cases. Because of the long incubation period of the disease, the liberation and spread of M. leprae into the environment during that stage constitute the main sources of infection. We searched for antigens that could be applied to the prediction of leprosy infection in subclinical cases.
Separation of proteins in one-dimensional electrophoresis is generally not sufficient to resolve a large number of constituents. 2-DE as described by O'Farrell (10) is very powerful since two different principles of protein separation are applied in the first and second dimensions, permitting a precise definition of individual antigens of samples. Proteins secreted by mycobacteria have been suggested as major immune targets in the early phase of infection (1). The CF of BCG was utilized to examine reactivities with sera from leprosy patients because cultivation of M. leprae has not yet succeeded in vitro. A study of crossreactivities with these proteins that contain well-characterized antigens may have potential value for finding new immunogenic antigens. The early cultures were used to exclude the possible contaminants with degraded products of bacteria.
In this study, we have defined three new antigens that are recognized by sera from leprosy and tuberculosis patients, the 29-, 24- and 23-kDa antigens (Fig. 2). The 29- and 24-kDa antigens were recognized very often in lepromatous patients but rarely in tuberculoid or TB patients. The reactions to these antigens can be good candidates for prediction of the infection, especially in lepromatous patients. Further study of these antigens may provide a better understanding of the pathogenesis.
Immunoblot analysis gave some patterns of reactivities with individual patient sera. There was a clear trend. Humoral response intensities against Ag85 complex and MPB51 were well correlated with the types of disease. The reactions with sera from lepromatous patients were stronger than those from tuberculoid and TB patients. Focusing on Ag85-B, an ELISA analysis was performed and yielded a similar conclusion (data not shown). Ag85 complex has recently been shown to be a mycolyl transferase that is one of the important enzymes for unique mycobacterial cell-wall synthesis (2). The Ag85 complex may play important roles as the first row antigens secreted by M. leprae after infection. The antigens are suggested to be the most principal ones for determining the clinical prognosis after infection.
Although relapse of lepromatous leprosy is a serious problem, this clinical change cannot be detected clearly in an early stage. Recently, multidrug-resistant M. leprae has been isolated from relapsing leprosy (3). Drug therapy for a long period to avoid relapse of the disease raises the possibility of selecting the drug-resistant M. leprae. The antibody responses to spots defined in this study might provide successful prediction for controlling the disease and for efficient therapy. Our findings may also contribute to the understanding of the pathogenesis of leprosy and the mechanism of the infection.
Acknowledgment. This work was partly supported by a grant l'rom the Human Science Foundation and by a grant-in-aid l'or Scicntilic Research (08771705) from the Ministry of Education, Science and Culture, Japan.
REFERENCES
1. ANDERSEN, P., ASKGAARD, D., GOTTSCHAU, A., BENNEDSEN, J., NAGAI, S. and HERON. I. Identification of immunodominant antigens during infec tion with Mycobacterium tuberculosis. Scand. J. Immunol. 36(1992)823-831.
2. BELISLE, J. T., VlSSA, V. D., SIEVERT, T., TAKAYAMA, K., BRENNAN, P. J. and BESRA, G. S. Role of the major antigen of Mycobacterium tu berculosis in cell wall biogenesis. Science 276(1997)1420-1422.
3. CAMBAU, E., PERANI. E., GUILLEMIN, I., JAMET, P. and Jl, B. H. Multidrug-resistanee to dapsone, rifampicin, and ofloxacin in Mycobacterium leprae. Lancet 349(1997)103-104.
4. DROWART, A., LAUNOIS, P., DE COCK M., HUYGEN, K., DE BRUYN, J., YERNAULT, J. C. and VAN VOOREN, J.-P. An isoelectric focusing method for the study of the humoral response against the anti gen 85 complex of Mycobacterium bovis BCG in the different forms of leprosy. J. Immunol. Meth ods 145(1991)223-228.
5. FILLEY, E., THOLE, J. P., R., ROOK. G. A. W., NAGAI. S., WATERS. M., DRIJFHOUT, J. W., RINKE DE WIT. T. F. DE VRIES, R. R. P. and ABOU-ZEID, C. Identification of an antigenic domain on Mycobac terium leprae protein antigen 85 B. which is specifically recognized by antibodies from pa tients with leprosy. J. Infect. Dis. 169(1994)162-169.
6. MATSUO, K., YAMAGUCHI, R., YAMAZAKI, A., TASAKA. H. and YAMADA, T. Cloning and expression of the Mycobacterium bovis BCG gene for extracellular alpha antigen. J. Bacterid. 170(1988)3847-3854.
7. MATSUO, T. MATSUO, H., OHARA. N., MATSUMOIO, S., KITAURA. H., MIZUNO, A. and YAMAHA. T. Cloning and sequencing of an MPB70 homologue corresponding to MPB83 from My cobacterium bovis BCG. Scand. J. Immunol. 43(1996)483-489.
8. NAGAI. S., NAGASUGA, T. and MATSUMOTO, J. Tu berculin peptide from culture filtrate of Mycobac terium tuberculosis. Am. Rev. Rcspir. Dis. 121(1980)551-557.
9. NAGAI. S., WIKER. H. G., HARBOE, M. and KINOMOTO. M. Isolation and partial characterization of major protein antigens in the culture fluid of Mycobacterium tuberculosis. Infect. Iiiunun. 59(1991)372-382.
10. O'FARRELL, P. H. High resolution two-dimensional electrophoresis of proteins. J. Biol. Chem. 250(1975)4007-4021.
11. OHARA. N., KITAURA, H., HOTOKEZAKA, H., NISHIYAMA. T, WADA, N., MATSUMOTO, S., MATSUO, T., NAITO, M. and YAMADA, T. Characterization of the gene encoding the MPB51, one of the major secreted protein antigens of Mycobacterium bovis BCG, and identification of the secreted protein closely related to the libronectin binding 85 complex. Scand. J. Immunol. 41(1995)433-442.
12. PESSOLANI, M. C. PERALTA, J. M., RUMJANEK, F. D., GOMES, H. M., MARQUES, M. A., ALMEDA, F. C. SAAD, M. H. and SARNO. F. N. Serology and leprosy: immunoassays comparing immunoglobulin G antibody responses to 28- and 30-kilodalton proteins purified from Mycobacterium bovis BCG. J. Clin. Microbiol. 29(1991)2285-2290.
13. RAMBUKKANA, A., DAS, P. K., BURGGRAAF, J. D., YONG, S., FABER, W. R., THOLE, J. F. R. and HARBOE, M. Heterogeneity of monoclonal antibody-reactive epitopes on mycobacterial 30-kilodaltonregion proteins and the secreted antigen 85 complex and demonstration of antigen 85B on the Mycobacterium leprae cell wall surface. Infect. Immun. 60(1992)5172-5181.
14. SHINNICK, T. M., VODKIN, M. H. and WILLIAMS, J. C. The Mycobacterium tuberculosis 65 kilodalton antigen is a heat shock protein which corresponds to common antigen and to the Escherichia coll groEL protein. Infect. Immun. 56(1988)446-451.
15. TERASAKA, K., YAMAGUCHI, R., MATSUO, K., YAMAZAKI, A., NAGAI. S. and YAMAHA. T. Complete nucleotide sequence of immunogenic protein MPB70 from Mycobacterium bovis BCG. FFMS Microbiol. Lett. 49(1989)273-276.
16. TOWBIN, H., STAEHELIN, T. and GORDON. J. Flectrophoretic transfer of proteins from polyaerylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. U.S.A. 76(1979)4350-4354.
17. VIKERFORS,T., OLCEN, P., WIKER, H. and WATSON, J. D. Serological response in leprosy and tuberculosis patients to the 18-kDa antigen of Mycobacterium leprae and antigen 85B of Mycobacterium bovis BCG. Int. J. Lepr. 61(1993)571-580.
18. YAMAGUCHI, R., MATSUO, K., YAMA/AKI, A., ABE, C, NAGAI. S., TERASAKA. K. and YAMADA, T. Cloning and characterization of the gene for immunogenic protein MPB64 of Mycobacterium bovis BCG. Infect. Immun. 57(1989)283-288.
19. YAMAGUCHI, R., MATSUO, K., YAMAZAKI. A., NAGAI, S., TERASAKA, K. and YAMADA, T. Immunogenic protein MPB57 from Mycobacterium bovis BCG: molecular cloning, nucleotide sequence and expression. FFBS Lett. 240(1988)115-117.
1 M. Naito, D.D.S., Ph.D., Department of Oral Bacteriology. Nagasaki University School of Dentistry. Sakamoto 1-7-1, Nagasaki 8528588, Japan.
2 S. Izumi, M.D., Ph.D., National Leprosarium Ohshima-Seisyouen, Kagawa, Japan.
3 T. Yamada. M.D., Ph.D., Department of Oral Bacteriology. Nagasaki University School of Dentistry. Sakamoto 1-7-1, Nagasaki 8528588, Japan.
Reprint requests to Dr. Yamada at the above address or PAX 81-95-849-7650; email: yamatake@net.nagasaki-u.ac.jp
Received for publication on 3 November 1997.
Accepted for publication in revised form on 27 February 1998.