- Volume 66 , Number 2
- Page: 214–7
Correlation of oral surface temperatures and the lesions of leprosy
ABSTRACT
A short review of the literature on the optimum temperature for the growth of Mycobacterium leprae is followed by a report of an investigation into the correlation of oral surface temperatures with oral leprous lesions. It is concluded that the oral lesions of leprosy occur more frequently in areas of the mouth with a lower surface temperature.RÉSUMÉ
Une brève revue de la littérature concernant la température optimale de croissance de Mycobacterium leprae est le point de départ d'une étude addressant la corrélation pouvant exister entre la température superficielle orale et les lésions orales de lèpre. Il est conclu que les lésions orales de lèpre apparaissent plus fréquemment dans des aires de la bouche caractérisées par une température superficielle plus basse.RESUMEN
Se hace una corta revisión de la literatura sobre la temperatura óptima para el crecimiento de Mycobacterium leprae y se reportan los resultados de una investigación sobre la correlación entre las temperaturas de la superficie oral y la presencia de lesiones orales de la lepra. Se concluye que las lesiones orales de la lepra ocurren más frecuentemente en aquellas áreas de la boca que tienen las temperaturas superficiales más bajas."The leprosy bacillus appears to be highly temperature dependent, for it produces lesions mainly in colder parts of the body . . ." (2).
The above statement is presently accepted by most leprologists as being true. In the first half of this century, the temperature theory, as stated above, was based mainly on clinical observations. In 1959, Brand gave an excellent summary of clinical observations favoring the temperature theory (5).
In a clinical study in 1968, Hastings, el al. strengthened the arguments in favor of the temperature theory by reporting that they found a significantly higher bacterial density in skin with a mean temperature of 32.05ºC when compared to skin with a mean surface temperature of 33.46ºC (l0). The study was based on temperature measurements as well as on skin biopsies taken from the lower back of five lepromatous leprosy patients. Shepard found Mycobacterium leprae to multiply most rapidly in mouse foot pads with a tissue temperature of 27ºC to 30ºC (17).
Franzblau and Harris showed the optimal temperature for synthesis of the M. leprae -specific phenolic glycolipid (PGL-I) to be 33ºC, and that ATP levels were higher with M. leprae cultures incubated at 33ºC or less (9).
This paper describes the findings of a clinical study using the oral cavity as a model to investigate the relationship between oral lesions of leprosy and oral surface temperatures. The oral cavity appears to be a more suitable model than skin since skin is exposed to the outside environment constantly compared to the occasional exposure of the oral mucosa.
MATERIALS AND METHODS
The frequency of distribution of oral leprous lesions as determined in a clinico-pathological study of 37 leprosy patients was plotted on a World Health Organization (WHO) topographical map, dividing the oral cavity into 44 different topographical locations (Fig. 1).
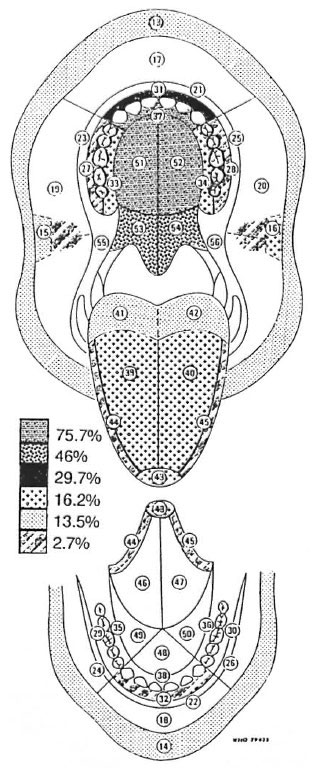
Fig. 1. Frequency of involvement of each WHO topographical location as a percentage of the number of patients with oral lesions.
Locations within the oral cavity affected by leprosy, in order of frequency, were the hard palate, followed by the soft palate, the labial maxillary gingiva, the tongue, the lips and, lastly, the buccal maxillary gingiva, the labial mandibular gingiva, and the buccal mucosa.
A percentage value for the frequency of leprous involvement of the mouth was allocated to each of the affected 44 WHO locations (Fig. 1). A full report of these results was published in 1993 (16).
The next part of the study was to correlate the frequency of distribution of the leprous lesions at the 44 WHO locations with the usual temperatures at these locations. An isotemperature contour map of the oral cavity was therefore sought after but no such map could be found in the literature.
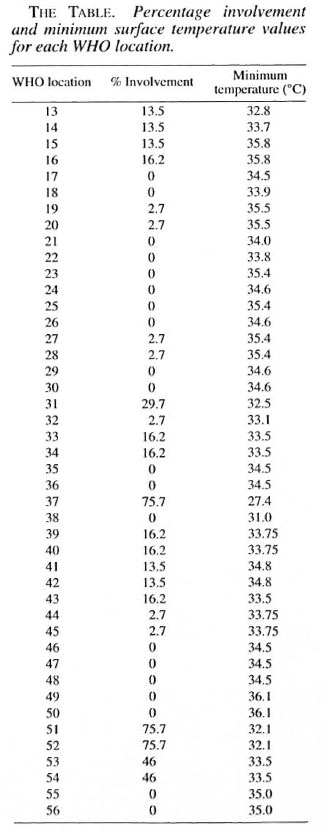
In 1970, Budylina, et al. (6) measured the surface temperatures of the oral structures of seven healthy, young adults at numerous locations using a thermo-couple under controlled conditions. Their figures were then compared to other less comprehensive studies, and a reasonable degree of correlation of results was found (4, 13, 19). This was confirmed by Spierings, et al . in their review paper (18). Budylina, et al . 's point-location temperatures were then transferred onto the WHO topographical contour map of the oral cavity by making use of a three-dimensional terrain model computer program. The temperature value of the lowest iso-temperature line crossing each of the 44 WHO areas was noted (The Table).
RESULTS
A minimum temperature value and a percentage value regarding the frequency of leprous involvement were obtained for each of the 44 WHO topographical areas. These values were then tabulated (The Table) and the two variables were tested for correlation using Pearson's correlation coefficient. The coefficient of variation (r) was found to be 0.66 (Fig. 2).
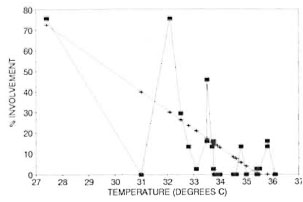
Fig. 2. Correlation between oral leprous involvement and oral surface temperature.  = actual: + = regression line.
= actual: + = regression line.
DISCUSSION
Temperature plays a prominent and unique role in the disease of leprosy. Peripheral leprous neuropathy is caused by invasion of the superficial peripheral nerves by leprosy bacilli. This reduces bloodflow, resulting in lower skin temperatures, especially of the extremities (1). These lower temperatures encourage the invasion and proliferation of M. leprae . By invading the cooler nasal mucosa, masses of M. leprae (105-107 organisms per day) are excreted in the nasal discharge (17). This causes dissipation of the bacilli into the environment, promoting the propagation of leprosy in the population. It has been demonstrated that the leprosy bacilli so excreted remain viable outside of the human body for more than 9 days (7).
The body temperature may be manipulated as a therapeutic measure. Progressive ulnar nerve destruction may be counteracted by placing the upper extremity in a cast (8). A tarsorrhaphy may be considered when confronted with a leproma increasing in size across the surface of the cornea (12). A tracheostomy will indirectly increase the temperature of the nasal mucosa, contributing to a reduction in the bacillary load (14, 15). The possible role of thyroxine to increase the basal metabolic rate has been proposed to raise the core temperature and to create an unfavorable high temperature for the proliferation of the bacilli (3).
The observation of leprosy bacilli in the liver parenchyma (a relatively warm organ) can be explained by the liver removing masses of bacilli from the bloodstream, and not as an invasion in the true sense of the word (5, 11). Heavy infiltration of M. leprae into the liver and spleen occurs in armadillos with their cool core temperature (14, l5).
With the exception of WHO location 38, till WHO locations having a surface temperature of less than 33.8ºC have some patients with lesions in these locations. The anterior lingual gingiva (WHO location 38) at only 31ºC, however, had no patients affected in this location. The relatively warm tongue almost constantly overlying this location may explain the absence of lesions.
The anterior palate (WHO location 37) was frequently involved (75.7%). This may be explained by its cool surface temperature of 27.4ºC. A contributory factor may be that bacilli may invade the palate directly from the heavily infiltrated nasal mucosa, especially via the nasopalatine duct.
Mouthbreathing is a very common finding in patients with lepromatous leprosy due to nasal involvement causing nasal stuffiness and obstruction (14, 15). The flow of cooler inspired air, as well as the cooling effect of evaporation, will certainly lower the surface temperature values, especially over the dorsum of the tongue and over the hard and soft palates. Unfortunately, no oral surface temperature values for mouth-breathers could be found in our literature search. For speculative reasons, cooler temperature values were allocated for the WHO locations corresponding to the dorsum of the tongue and palate. Postulating cooler surface temperature values of 3ºC and 7ºC below the actual values in these locations just marginally increased the correlation coefficients to 67 and 67.4, respectively. It should be borne in mind that the temperature values were taken in non-mouth-breathers, but with an open mouth; thus, to some extent simulating the effects of mouthbreathing (6).
This study shows that the oral lesions of leprosy occur more frequently in areas of the mouth with lower surface temperatures. In light of the literature cited, it is concluded that leprosy bacilli proliferate in sites having a temperature lower than normal core body temperature.
REFERENCES
1. ABBOT, N. C. BECK, J. S., SAMSON. P. D., BUTLIN, C. R., BENNETT, P. J. and GRANGE, J. M. Cold lingers in leprosy. Int. j. Lepr. 60(1992)580-586.
2. ANDERSON, J. R. Muir's Textbook of Pathology . 10th edn. London: Edward Arnold Publishers Ltd., 1976, pp. 180-181.
3. ANNAL, N. Thyroxine may cure leprosy, fungal diseases and metabolic cataract. Med. Hypotheses 35(1991)315.
4. BERGSTROM, J. and VARGA, G. Temperatures of the oral cavity in 50 healthy students. Swed. Dent. J. 64(1971)157-164.
5. BRAND, P. W. Temperature variation and leprosy deformity. Int. J. Lepr. 27(1959)1-7.
6. BUDYLINA, S. M., KOLESNIKOV, L. L. and POLJAKOV. V. V. The topography of temperature indices of the oral cavity. Stomatologica (Moscow) 49(1970)76-78.
7. DESIKAN, K. V. Viability of Mycobacterium leprae outside the human body. Lepr. Rev. 48(1977)231-235.
8. ENNA, C. D., BERGHTHOLDT, H. T. and STOCKWELL, E. A study of surface and deep temperatures along the course of the ulnar nerve in the pisohamate tunnel. Int. J. Lepr. 42(1974)43-47.
9. FRANZBLAU, S. G. and HARRIS, E. B. Biophysical optima lor metabolism of Mycobacterium leprae . J. Clin. Microbiol. 26(1988)1124-1129.
10. HASTINGS, R. C, BRAND, P. W, MANSFIELD, R. E. and EBNER, J. D. Bacterial density in the skin in lepromatous leprosy as related to temperature. Lepr. Rev. 39(1968)71-74.
11. JON, C. K., CHEHL, S., HASTINGS, R. C. and RUBY, J. R. Invasion of liver parenchymal cells by Mycobacterium leprae in an experimentally infected nude mouse. Am. J. Trop. Med. Hyg. 32(1983)1088-1095.
12. LEWALLEN, S. Prevention of blindness in leprosy: an overview of the relevant clinical and programme-planning issues. Ann. Trop. Med. Para-sitol. 91(1997)341-348.
13. MAEDA, T., STOLTZE. K., USER, A., KROONE, H. and BRILL, N. Oral temperatures in young and old people. J. Oral. Rehabil. 6(1979)159-166.
14. MEYERS, W. M., GORMUS, B. J. and WALSH, G. P. Experimental leprosy. In: Leprosy . 2nd edn. Hastings. R. C, ed. Edinburgh: Churchill Livingstone. 1994, pp. 391-392.
15. PFALTZGRAFF, R. E. and RAMU, G. Clinical leprosy. In: Leprosy . 2nd edn. Hastings, R. C. ed. Edinburgh: Churchill Livingstone, 1994, pp. 268-270.
16. SCHEEPERS, A., LEMMER, J. and LOWNIE:, J. E. Oral manifestations of leprosy. Lepr. Rev. 64(1993)37-43.
17. SHEPARD, C. C. Temperature optimum of Mycobacterium leprae in mice. J. Bacteriol. 90(1965)1271-1275.
18. SPIERINGS, T. A. M., PETERS, M. C. R. B. and PLASSCHAERT, A. J. m. Surface temperatures of oral tissues; a review. J. Biol. Bucc. 12(1984)91-99.
19. VOLCHANSKY, A., CLEATON-JONES, P., WRIGHT, P. G. and FATTI, L. P. Gingival and labial vestibular temperature in young individuals. J. Dent. 13(1985)323-330.
A. Scheepers, B.Ch.D., M.Dent., F.F.D., Department of Maxillofacial and Oral Surgery, Oral and Dental Teaching Hospital, University of Witwatersrand, Johannesburg, South Africa.
Reprint requests to Dr. A. Scheepers, P.O. Box 146209, Bracken Gardens 1452, South Africa.
Received for publication on 21 March 1997.
Accepted for publication in revised form on 22 April 1998.