- Volume 66 , Number 2
- Page: 131–9
Epidemiological significance of first skin lesion in leprosy
ABSTRACT
The epidemiological significance of monolesions in leprosy and the possible in ferences on the mode of entry by Mycobacterium leprae into the body are presented based on data f rom the clinical records of the Leprosy Control Programme of Gudiyatham Taluk in India; 660 children with monolesions (335 males, 305 females) younger than 15 years of age and detected during the period 1990-1995 were included in the study. Detailed investigations on the location of monolesions were carried out and compared with a random sample of 669 normal rural children matched for age and sex. A large majority of the leprosy monolesions were in the uncovered parts of the body, with special predilection for the posterior aspects of the upper extremities and the anterior aspects of the lower extremities. Based on observation of normal children, these happen to be precisely the sites vulnerable for trauma since they are exposed to the environment where M. leprae could enter through abraded skin and manifest as a patch. The need for further studies is emphasized.RÉSUMÉ
La signification épidémiologique des monolesions et les possibles déductions que l'on peut formuler quant à la voie d'entrée de Mycobacterium leprae dans l'organisme sont présentées à partir des données provenant des dossiers cliniques du Programme de Contrôle de la Lèpre de Gudiyatham Taluk en Inde: 660 enfants affectés de monolésions (335 garçons et 305 filles), de moins de 15 ans, détectés entre 1990 et 1995, lurent inclus dans l'étude. Une recherche détaillée de la localisation des monolésions fut entreprise et comparée à un échantillon témoin de 669 enfants ruraux normaux d'âge et de sexe comparables. La majorité des monolésions de lèpre était située sur les parties non habillées du corps, avec une nette tendance de localisation à la face postérieure de l'extrémité des bras et la face antérieure de l'extrémité des jambes. Basé sur l'observation d'enfants normaux, ces sites correspondent aux sites les plus courants d'exposition aux blessures. Comme ces sites correspondent précisément aux régions susceptibles aux blessures, ces résultats impliquent la possibilité de transmission de M. Leprae à partir de l'environnement, à travers une peau lésée et le développement d'une macule localement. Nous soulignons le besoin d'entreprendre des études plus poussées à partir de ces résultats.RESUMEN
Se hizo un estudio basado en los datos de los expedientes clínicos del Programa de Control de la Lepra de Gudiyatham Taluk, India, sobre la significancia epidemilógica de las lesiones únicas (monolesiones) de la lepra y sobre el modo de entrada de Mycobacterium leprae en el cuerpo; en el estudio se incluyeron 660 niños menores de 15 años con monolesiones (335 hombres. 305 mujeres) detectados en el periodo de 1990 a 1995. Se hicieron investigaciones detalladas sobre la localización de las monolesiones y los resultados se compararon con los encontrados en una muestra tomada al azar de 669 niños rurales, de edad y sexo similares. La gran mayoría de las monolesiones de la lepra estuvieron en las partes descubiertas del cuerpo y mostraron una especial predilección por la superficie posterior de las extramidades superiores y la superficie anterior de las extremidades inferiores. En los niños en general, estos sitios son precisamente los más vulnerables a traumas y por esto constituyen sitios propicios para la entrada de los bacilos de la lepra quienes al penetrar a través de piel lesionada dan origen a las lesiones maculares de la enfermedad. Se enfatiza la necesidad de estudios adicionales sobre el tema.Many epidemiological features of leprosy, including the mode of entry of Mycobacterium leprae into the body are still not definitely known. Careful studies of sites of first lesions have held some promise in this aspect (20, 29, 31, 35, 36), lending credence to a number of possible hypotheses. Based on studies in southern India, Horton and Povey (12) conclude that the distribution of first lesions is nonrandom, confined to exposed parts of the body and they support the hypothesis of entry through the skin. Bechelli, el al. (2) investigated 469 patients with single lesions in Myanmar (Burma) and conclude that although early lesions are concentrated over the elbows and knees it is unlikely such lesions develop at the point of entry of M. leprae since these areas are general^ clothed. In a study of leprosy among an African population in Malawi (24), the anatomical distribution of single lesions did not provide enough evidence for association with the nervous or vascular system, and the authors concluded that the distribution reflects the influence of some "local" environmental or behavioral factors. Although the skin-to-skin contact has been relegated to a minor position by strong proponents of the naso-respiratory mode of transmission (21, 27), the appearance of skin lesions, especially the first ones, have continued to challenge scientists to examine the portal of entry more carefully.
We have carried out a thorough investigation on the location of patches in leprosy among children younger than 15 years of age detected with monolesions during 1990-1995. We present the relevant data and discuss possible inferences on the mode of entry and the epidemiological significance of the appearance of the first patch in leprosy.
MATERIALS AND METHODS
These studies were carried out in Gudiyatham Taluk, Vellore District, Tamil Nadu, India, known to be hyperendemic for leprosy. The people and living conditions in this taluk are typical of that prevailing in other rural areas in Tamil Nadu and have been described elsewhere (13, 24).
The entire population was periodically enumerated and screened for new cases through many surveys by paramedical workers/nonmedical supervisors and referred to the village clinic for confirmation of the diagnosis. A general survey was done by screening the entire population once in 5 years; a contact survey and a school survey were done once a year. Women trained in leprosy detection work were utilized, and cases were also detected constantly through the voluntary reporting from the input of health education. Qualified physiotherapy technicians were utilized in motor-sensory assessment and in screening for peripheral nerve thickening. Each patch was carefully examined for loss of sensation, pain sensitivity and thermal sensitivity, using the following methods.
First, the patients were made to sit at ease, and the procedure was explained and demonstrated with their eyes open. After they had understood they were asked to close their eyes. The normal skin was touched with a leather and the patient was asked to indicate the site touched by his index finger. When they responded, the suspected skin lesion was touched next, and the response was registered. The skin was touched alternatively with the sharp and the blunt end of a pin. The patient was asked to tell whether the feeling was sharp or dull. A thermal sensation tester was adopted to elicit loss of hot/cold temperature sensation. All cutaneous and nerve trunks were examined thoroughly and compared with the opposite side. Cases who were doubtful, not classical in presentation, or who failed to satisfy any of the cardinal signs were kept under observation and reviewed and/or biopsied. A diagnosis of leprosy was confirmed by the examining physician upon detection of a hypopigmented skin lesion with definite loss of sensation. The patients were clinically classified according to the standard Ridley-Jopling classification (28). Smears were taken from four different routine sites: right ear, left forehead, right chin and left buttock or thigh and over the patch.
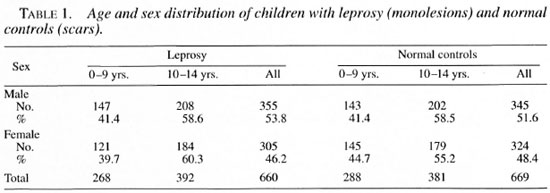
During the years 1990-1995 a total of 794 children below the age of 15 were diagnosed as having leprosy. Of these, 660 children, 355 (53.8%) males and 305 (46.2%) females, who had monolesions were included in the study: 282 (43%) were detected through a school survey (SS), 180 (27%) through a general survey (GS), 141 (21%) through voluntary reporting (VR) and 57 (9%) through a contact survey (CS). Seventy-eight (12%) belonged to the indeterminate group, 373 (56%) were borderline tuberculoid (BT), and 209 (32%) polar tuberculoid; one child was found to be selective site-smear positive for acid-fast bacilli (AFB) with a bacterial index (BI) of 2+, later confirmed as borderline tuberculoid by histopathological examination. The age and sex distribution of the 660 patients are shown in Table 1.
A random sample of 669 normal children in three villages (matched for age and sex distribution of children with monolesions) were questioned and examined thoroughly for scars, abrasions and fresh sores on the skin. Scars were defined as those visible to the naked eye, caused by any injury and excluded birthmarks. The numbers and distribution of scars according to location were noted and compared with those of the leprosy lesions.
In order to test the ranking of the anatomical sites for monolesions in leprosy patients and scars in the normal controls, Spearman's rank correlation coefficient was used. Rejection of a null hypothesis implies a statistically significant correlation and, hence, similarity of ranking in the two groups.
FINDINGS
The locations of leprosy monolesions on the body are given in Table 2 and shown in The Figure. The distribution revealed that 240 (36%) were located on the upper extremity, 249 (38%) on the lower extremity, 33 (8%) on the trunk, and 118 (18%) on the face.
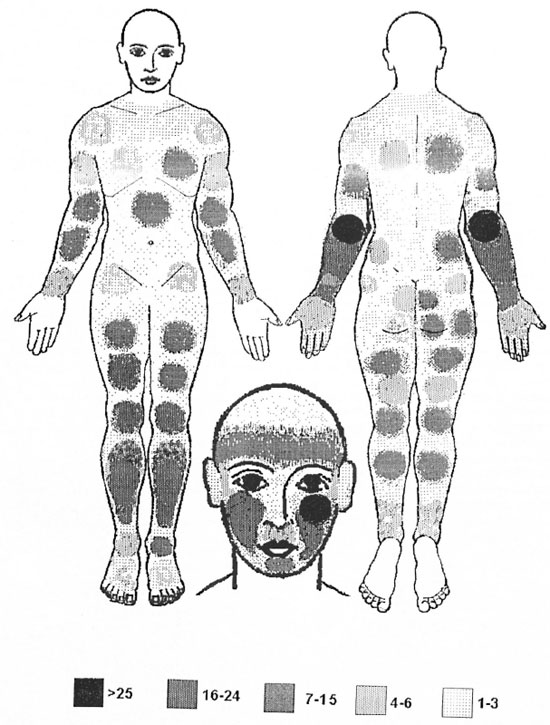
The Figure. Number of children showing monolesions by site.
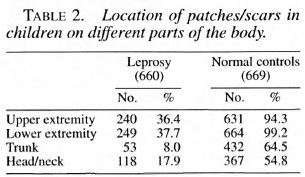
The distribution of monolesions on thedifferent regions of the upper extremity inthe order of magnitude were as follows:forearm 160, hand 46, upper arm 27, shoulder 7 (Table 3); on the lower extremity = leg 109, thigh 82, buttock 38, feet 20 (Table 4); on the trunk = thorax 27; loins 15; ab-domen 7, flank 4 (Table 5); and on the face= cheek 79, forehead 18, chin 9, lip 4, neck4, nose 3 and ear I (Table 6).
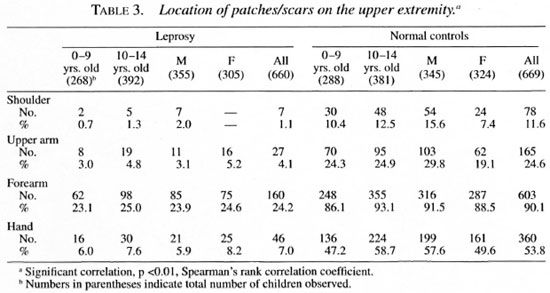
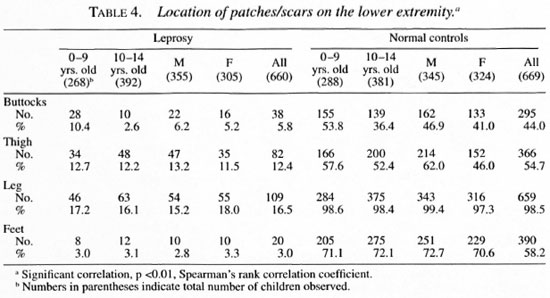
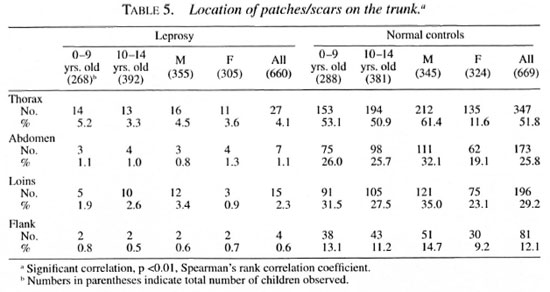
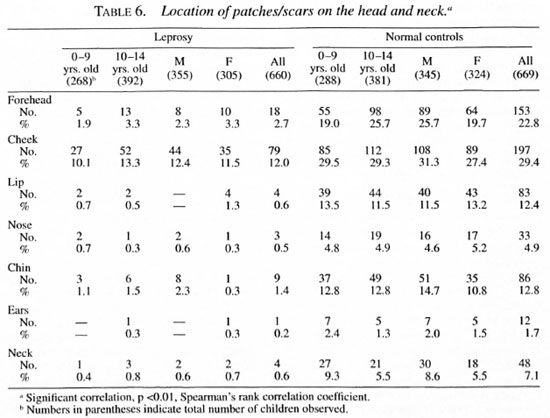
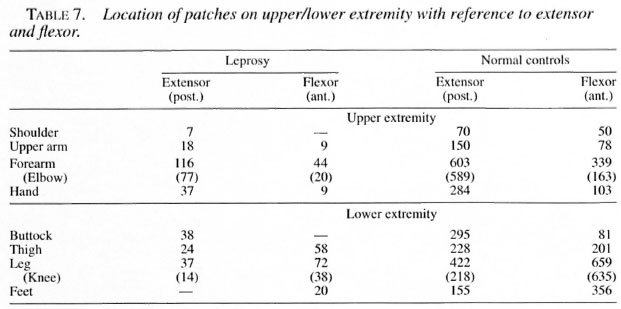
Seventy-seven children had monolesionsover the extensor elbow whereas only 20 ofthem had lesions over the cubital fossa. Likewise, 38 had monolesions on the kneeand only 14 over the popliteal fossa. The locations of the patches in the extensor (posterior)/flexor (anterior) aspect of the upperextremities and extensor (anterior)/flexor(posterior) aspect of lower extremities areshown in Table 7.
The distribution of scars on the 669 normal children examined revealed 631 (94.3%)on the upper extremity, 664 (99.2%) on thelower extremity, 432 (64.5%) on the trunkand 367 (54.8%) on the face.
The distribution of scars on the differentregions of the upper extremity in order ofmagnitude were as follows: forearm 603,hand 360, upper arm 165, shoulder 78(Table 3); on the lower extremity = leg 659, feet 390, thigh 366, buttock 295 (Table 4); on the trunk = thorax 347, loins 196, abdomen 173, flank 81 (Table 5); and on theface = cheek 177, forehead 153, chin 86, lip83, neck 48, nose 33 and ear 12 (Table 6).
Ninety-three percent of the children hadscars over the elbows whereas only 39% ofthem had scars over the cubital fossa. Like-wise, 98.6% had scars over the knees andonly 41.4% had scars over the popliteal fossa. Similarly, scars were commonly seenover the extensor aspect and rarely over theflexor regions of both limbs. The distribution of scars in the extensor and flexor aspect of the upper and lower extremities areshown in Table 7.
On detailed history, it was noted that thescars were mostly due to scratches by sharpobjects, after a fall, and an ulcer followingsecondary infections. Scratches due to pruritis of various causes with uncut fingernails and superimposed secondary infection further worsened the breech of the integument. Fissures were present over the angles of the mouth due to vitamin deficiency and/or nasal excoriation following repeated cleaning of discharge with rough material. Occasionally, they were also due to burns, dog bite and tattooing.
The scalp of newborn children which was shaved repeatedly during their childhood would inevitably result in small cuts and nicks. Branding and boring of ears are rituals practiced in this area. Almost every child inevitably had a scar/wound over the knees and elbows. Scars of traumatic origin were mainly seen over the exposed areas and scars of infectious origin, i.e., scabies, impetigo, eczema, were mainly seen over the intertrigenous areas. These details are useful in interpreting possible entry of M. leprae into the human host.
DISCUSSION
It is now well accepted that a hypopigmented anesthetic patch is the first and earliest lesion in leprosy, except in pure neural cases, and it is often found in parts of the body which are not covered by clothes (8, 18, 30). In a study of 469 children with monolesions (2) in Burma, 87% were seen on the lower and upper limbs and face and only 12.6% were seen on the trunk. The knees and elbows were selectively affected. It should be noted, however, that in that report only after the age of 5 years did the children wear clothes to cover a major portion of the body (2). A report from north Malawi stated that in all age groups there was an excess of lesions relative to the surface area on the face, back of trunk, back of arms, front of legs and buttocks (520/635)(24). In our present study, only 8% (53) out of 660 had lesions on the trunk. It is interesting to note that 38 lesions were found on the anterior aspect of the knee and 77 on the posterior aspect of the elbow, a disproportionately high number considering their relative surface areas.
Unlike that seen among adults, where there is an excess of males, the sex ratio among children is nearly the same in this cohort as also observed earlier (26). Our survey revealed that children younger than 5 years often were poorly fed and clothed. In summer, clothing was meager, if not absent. From 5 to 10 years the girls were apparently better clothed than boys. After 10 years of age the girls were covered from the neck down to the knee (3) but the boys had only short pants, except during winter.
Exposed parts of a child's body are liable to enormous injuries, both minor and major, almost from infancy. Pre-adolescent/adolescent boys who are less covered had more scars than the girls of the same age group.
Our finding that the majority of the lesions were found on the lower and upper extremities is in agreement with that of Bechelli, et al. (2) , but does not compare with the distribution of lesions reported by Ponnighaus et al. (24), in whose study a large majority of the lesions were on the face. However, the selective involvement of the posterior aspect of the arms and the anterior aspect of the legs and buttocks reported by them agrees with our findings and other similar studies (1, 2). It is clear from our study and other similar studies (3) that a large majority of the lesions are in the uncovered parts of the skin with a special predilection for the posterior aspect of the upper extremities and the anterior aspect of the lower extremities and, to a lesser extent, the face. In daily activities these areas come in closer contact with the environment than do other parts of the body, and they are also more likely to be traumatized as compared with the anterior aspect of the upper limbs and the posterior aspect of the lower limbs. The knees and the elbows are especially vulnerable in this regard (4, 16).
The frequency of early lesions at these sites can be explained in two ways: a) Since these sites are in close and constant contact with the dusty and dirty environment where M. leprae survive up to 46 days (6), the organisms could enter the body at these sites through abrasions following trauma (16, 21). These sites have a low body temperature suitable to support the growth of the organisms (11) in susceptible persons (7, 37). Leprosy lesions following accidental inoculation (23), tattooing (25, 32), dog bite (10), vaccination (34) and thorn prick (15) also have been documented, b) Secondly, the organisms could enter the body through some other sites, such as the sole of the foot (97% of the children in the village walk barefoot) or through the nose (5, 21) . They are then taken up by the macrophages and circulate in the blood in susceptible persons, in whom local environmental factors (such as temperature at the site of infection, trauma and other hitherto undiscovered factors) may be responsible for localizing the circulating M. leprae at the site of the first lesion (14, 17). Of these two possibilities we suggest that the first one as the most likely, i.e., the point of entry being the site of the initial lesion.
Further studies are needed to carefully document the history and site of leprosy patches. As transmission of leprosy gets reduced, more intensive research will be required to elucidate the epidemiology of leprosy.
Acknowledgment. The authors are thankful to the Karigiri Research Committee for valuable suggestions. We also acknowledge assistance from Mr. Y. Sathiyaraj (physiotherapist), Mr. J. Anandaraj (Medical Records Department) and Mr. Lewis Kumar (secretarial help).
REFERENCES
1. AVELLEIRA, J. C. R., VLANNA, R. F., COUTINHO, R. B., MARQUK'S BOECHAT, A. and GOMES DI-; ANDRADE, V. R. Distribution of single cutaneous lesions in paucibacillary leprosy. Acta Leprol. 8(1993)127-131.
2. BECHELU, L. M., GARBAJOSA, G. P., GYI, M. G, DOMINGUEZ, V. M. and QUAGUATO, R. Site of early lesions in children with leprosy. Bull. WHO 48(1973)107-111.
3. Bedi, B. M. S., Narayanan, E., Doss, A. G., Kurchheimer, W. F. and Balasubrahmanyan, M. Distribution of single lesion of tuberculoid leprosy. Lepr. India 47(1975)15-18.
4. CHATURVEDI, R. M. Epidemiological study of leprosy in Malwani suburb of Bombay. Lepr. Rev. 59(1988)113-120.
5. ChEHL, S., JOB, C. K. and HASTINGS, R. C. Transmission of leprosy in nude mice. Am. J. Trop. Med. Hyg. 34(1985)1161-1166.
6. DESIKAN, K. V. Viability of Mycobacterium leprae outside the human body. Lepr. Rev. 48(1977)231-235.
7. DE VRIES, R. R., MLTIRA, N. K., VAIDYA, M. C, GUPTA, M. D., KHAN, P. M. and VAN ROOD, J. J. HLA linked control of susceptibility to tuberculoid leprosy. Tissue Antigens 16(1980)294-304.
8. DOUIX, J. A., RODRIGUEZ, J. N., GUINTO, R. and PLANTILLA, F. C. A field study of leprosy in Cebu. Int. J. Lepr. 4(1936)141-170.
9. GANAPATI, R., NAIK, S. S. and PANUYA, S. S. Childhood leprosy; study of prevalence rates and clinical aspects through surveys in Bombay. Lepr. India 48(1976)645-660.
10. GUPTA, C. M., TUTAKNE, M. A., TIWARI, V. D. and CHAKRABARTY, N. Inoculation leprosy subsequent to dog bite; a case report. Indian J. Lepr. 56(1984)919-920.
11. HASTINGS, R. C, BRAND, P. W., MANSFIELD, R. E. and EBNER, J. D. Bacterial density in the skin in lepromatous leprosy as related to temperature. Lepr. Rev. 39(1968)71-74.
12. HORTON, R. J. and POVEY, S. The distribution of first lesions in leprosy. Lepr. Rev. 37(1966)113-114.
13. JESUDASAN, K., VIJAYAKUMARAN, P., MANIMOZHI, N., RAJA, S., BUSHANANI, J. R., KANAGARAJAN, S. and SUNDAK RAO, P. S. Origin of new leprosy cases during general surveys in relation to previous survey findings. Lepr. Rev. 67(1996)183-189.
14. JON, C. K., BASKARAN, B., JOSEPH, J. and ASCHHOIT, M. Histopathologic evidence to show that Indeterminate leprosy may be a primary lesion of the disease. Int. J. Lepr. 65(1987)443-449.
15. JON, C. K., HARRIS, E. B., ALLEN, J. L. and HASTINGS, R. C. Thorns in armadillo ears and noses and their role in the transmission of leprosy. Arch. Pathol. Lab. Med. 110(1986)1025-1028.
16. JON, C. K., KAHAKONEN, M. E., JACOBSON, R. R. and HASTINGS, R. C. Single lesion subpolar lepromatous leprosy and its possible mode of origin. Int. J. Lepr. 57(1989)12-19.
17. JOB, C. K., SANCHEZ, R. M., MCCOKMICK, G. T. and HASTINGS, R. C. First lesion in experimental armadillo leprosy. Indian J. Lepr. 57(1985)71-77.
18. LARA, C. B. and DE VERA, B. Clinical observations with reference to leprosy in children of lepers. J. Philipp. Med. Assoc. 15(1935)115-129.
19. LARA, C. B. and DE VERA, B. Early leprosy in infants born of leprous patients; with report of cases. J. Philipp. Med. Assoc. 15(1935)252-267.
20. Levan, N. E., Bystryn, J. C. and Hyman, C. Temperature and blood flow in macules of lepromatous leprosy. Int. J. Lepr. 37(1969)249-252.
21. MACHIN, M. The mode of transmission of leprosy. In: Essays on Leprosy by Oxford Medical Students. Ryan, T. J. and McDougall, A. C, eds. Oxford: Department of Dermatology. The Slade Hospital, 1983, pp. 1-29.
22. MACHIN, M. A possible mode of entry to the body of Mycobacterium leprae. Lepr. Rev. 59(1988)87-89.
23. MARCHODX, P. E. A case of leprosy due to accidental inoculation. Int. J. Lepr. 2(1934)1-6.
24. PONNIGHAUS, J. M., FINE, P. E. M., GRUER, P. J. K. and MAINE, N. The anatomical distribution of single leprosy lesions in an African population, and its implications for the pathogenesis of leprosy. Lepr. Rev. 61(1990)242-250.
25. PORRITT, R. J. and OSIEN, R. S. Two simultaneous cases of leprosy developing in tattoos. Am. J. Pathol. 23(1947)805-817.
26. RAO, P. S. S., KARAT, A. B. A., KAUPERUMAL, V. G. and KARAT, S. Transmission of leprosy within households. Int. J. Lepr. 43(1975)45-54.
27. REES, R. J. and MEADE, T. W. Comparison of the modes of spread and the incidence of tuberculosis and leprosy. Lancet 1(1974)47-49.
28. RIDLEY, D. S. and JOPUNG, W. H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966)255-273.
29. RODRIGUEZ, J. N. Studies on early leprosy in children of leprosy patients. Philipp. J. Sci. 31(1926)115-144.
30. ROGER, L. and MUIR, E. Leprosy. Bristol: John Wright and Sons, 1925, p. 158.
31. SABIN, T. D. and EISNER, J. D. Patterns of sensory loss in lepromatous leprosy. Int. J. Lepr. 37(1969)239-248.
32. SEGHAL, V. N. Inoculation leprosy appearing after seven years of tattooing. Dermatológica 142(1971)58-61.
33. SEGHAL, V. N. and CHAUDHRY, A. K. Leprosy in children; a prospective study. Int. J. Dermatol. 32(1993)194-197.
34. SEGHAL, V. N., REGE, V. L. and VEDIRAJ, S. N. Inoculation leprosy subsequent to small-pox vaccination. Dermatológica 141(1970)393-396.
35. SPICKETT, S. G. Letters to the Editor. Lepr. Rev. 34(1963)154-154.
36. SUSMAN, L. A. A limited investigation into the significance of the site of the first lesion in leprosy. Lepr. Rev. 38(1966)37-42.
37. VAN EDEN, W., GONZALEZ, N. M, DE VRIES, R. R. P., CONVRR, J. and VAN ROOD, J. J. HLA-Linked control of predisposition to lepromatous leprosy. J. Infect. Dis. 151(1985)9-14.
1. S. Abraham, M.B.B.S., Medical Officer.
2. N. Mani Mozhi, M.B.B.S., D.H.E., Epidemiologist.
3. G. A. Joseph, M.D., Community Health Specialist, Branch of Epidemiology and Leprosy Control.
4. N. Kurian, M.Sc, Biostatistician, Department of Biostatistics.
5. P. S. S. Sundar Rao, Dr.Ph., Head, Branch of Epidemiology and Leprosy Control and Director, Schieffelin Leprosy Research and Training Centre, Karigiri 632 106, Vellore District, Tamil Nadu, India.
6. C. K. Job, M.D., E.R.C.Path., Consultant Pathologist, St. Thomas Hospital, Chettupattu, India.
Reprint requests to Dr. P. S. S. Sundar Rao, Director, SLR&TC, Karigiri 632 106, Vellore District, Tamil Nadu, India.
Received for publication on 6 April 1998.
Accepted for publication in revised form on 6 May 1998.