- Volume 66 , Number 2
- Page: 140–8
Spontaneous leprosy in a wild-caught cynomolgus macaque
ABSTRACT
Naturally occurring Mycobacterium leprae has been previously documented in only two species of nonhuman primates f rom West Africa-the chimpanzee and the sooty mangabey. We report here the first known case of spontaneous leprosy in an Asian macaque. A wild-caught cynomolgus macaque imported f rom The Philippines developed a reaction to a tuberculin skin test after 3 years at the California Regional Primate Research Center (CRPRC), University of California-Davis, Davis, California, U.S.A. Biopsies of concurrent skin lesions suggested a cutaneous mycobacterial infection. Diagnosis of the infection was obtained by a polymerase chain reaction (PCR) assay specific for M. leprae. Clinical presentation, histopathologic findings, and ELISA serology for M. leprae-specific PGL-I and to the LAM mycobacterial antigens were consistent with those of human borderline (BB) leprosy. Longitudinal serologic data suggest that the cynomolgus macaque had subclinical leprosy at the time of arrival in the CRPRC quarantine. Intradermal tuberculin testing is the traditional method for screening nonhuman primate populations for mycobacterial infections. Exposure to nontuberculous mycobacteria, such as M. leprae, may sensitize some individual primates to nonspecific mycobacterial antigens, resulting in false-positive tuberculin reactions. Susceptibility of the cynomolgus macaque and other nonhuman primates to M. leprae should be re-evaluated. Cynomolgus macaques and, possibly, other nonhuman primates may serve as valuable experimental models of leprosy in humans.RÉSUMÉ
L'infection naturelle par Mycobacterium leprae n'a été documentée que chez deux espèces de primates de l'Afrique de l'Ouest-le chimpanzé et le mangabey cendré. Nous rapportons ici le premier cas connu de lèpre spontanée chez un macaque asiatique. Un macaque Cynomolgus importé des Phillipines a développé une réaction positive au test à la tuberculine après 3 années passées au Centre Régional Californien de Recherche sur les Primates (CRPRC), Université de Californie-Davis à Davis. Californie, F.tats Unis d'Amérique. Des lésions cutanées étaient présentes chez cet animal. Ces lésions, qiu lurent biopsiées, suggéraient une infection cutanée à mycobactéries. Le diagnostic de certitude fut obtenu à l'aide de la Réaction de Polymerase en Chaîne (PCR) spécifique de M. leprae. La présentation clinique, les résultats histopathologiques et la sérologie par FLISA de l'antigène spécifique de M. leprae PGL-1 et de l'antigène myeobactérien LAM étaient compatibles avec ce qui est observé chez l'homme présentant la forme borderline (BB) de la lèpre. Les données sérologiques longitudinales suggèrent que ce macaque Cynomolgus souffrait de lèpre inapparente à son arrivée en quarantaine au CRPRC. Le test intra-dermique à la tuberculine est la méthode traditionnelle de dépistage des populations simiennes contre les infections à mycobactéries. L'exposition à des mycobactéries autres que le bacille tuberculeux, comme M. leprae, peuvent sensibiliser certains singes à des antigènes communs mycobactériens non spécifiques, dont le résultat est un test faussement positif à la tuberculine. La susceptibilité des macaques Cynomolgus ainsi que d'autres espèces de singes devraient être reconsidérée. Les macaques Cynomolgus et probablement d'autres espèces de singes pourraient s'avérer être d'utiles modèles expérimentaux de la lèpre humaine.RESUMEN
La infección natural por Mycobacterium leprae en primates no humanos sólo se ha documentado en el chimpancé y en el mono mangabey cenizo en el Africa Occidental. Aquí reportamos el primer caso de lepra espontánea en un macaco asiático. Un macaco cinomolgo silvestree importado de las Filipinas desarrolló una reacción dérmica a la tuberculina después de 3 años de cautiverio en el Centro Regional para la Investigación de Primales de California (CRPRC) de la Universidad de California-Davis, Davis, CA, U.S.A. Las biopsias de las lesiones de la piel concurrente sugirieron una infección micobacteriana cutánea. F.l diagnóstico de la enfermedad se obtuvo mediante la técnica de la reacción en cadena de la polimerasa (PCR) específica para M. leprae. Los datos clínicos, los hallazgos histopatológicos, y las pruebas de FLISA con el PGL-I específico de M. leprae y con Iipoarabinomanana (LAM) indicaron una forma de lepra intermedia (BB). Los datos serológicos longitudinales sugirieron que el macaco cinomolgo tenía una infección leprosa subelíniea en el tiempo de su arribo al CRPRC. La prueba intradérmica con tuberculina es el método tradicional para buscar infecciones micobacterianas en primates no humanos. La exposición de estos animales a inicobacterias no tuberculosas tales como M. leprae, puede sensibilizar a algunos primates hacia antígenos mieobacterianos no especilicos ocasionando reacciones falsas-positivas a la tuberculina. La susceptibilidad del macaco cinomolgo y de otros primates no humanos a M. leprae debe ser reevaluada ya que estos animales podrían resallar de gran valor como modelos experimentales de la lepra en los humanos.Naturally acquired leprosy has been previously documented in two species of non human primates from West Africa: the chimpanzee (Pan troglodytes) (6, 9) and the sooty mangabey (Cercocebus atys) (8, 23, 24). In this paper we describe the clinical presentation, ELISA serology, and histopathological findings of naturally acquired leprosy in a cynomolgus macaque (Macaca fascicularis). This is the first reported case of spontaneous leprosy in an Asian nonhuman primate species.
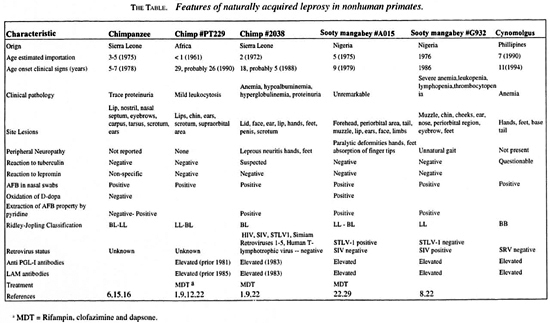
The host immune response to Mycobacterium leprae is critical for control of the infection. This response, though, results in the iinmunopathological damage to skin and nerves that is the basis for the clinical spectrum of disease (27). At one pole of the spectrum is tuberculoid leprosy (TT), as classified by Ridley and Jopling. Patients with TT have a strong lymphocytic infiltrate and an effective histiocytic response to M. leprae, resulting in well-localized granulomas and few surviving organisms (27). The tubercles or granulomas in TT leprosy are typical of the chronic delayed-type (type IV) hypersensitivity (DTH) response, and are composed of activated epithelioid macrophages surrounded by a dense population of T cells. Among the T cells, the CD4+ helper phenotype predominates and is scattered diffusely, while CD8+ T cells are limited to the outer mantle of the epithelioid granuloma (2, 14). At the other end of the spectrum is lepromatous leprosy (LL), in which patients exhibit a selective unresponsiveness to M. leprae antigens. The immunologic response is relatively weak; composed of a few scattered lymphocytes, the CD8+ subset predominating (20), and an ineffective, diffuse histiocytic reaction with numerous intralesional organisms. Between these two polar groups lies a broad variety of borderline forms: borderline tuberculoid (BT), mid-borderline (BB), and borderline lepromatous (BL). These intermediate forms describe a progressive reduction in the cellular-immune response which is accompanied by an increasing number of skin and nerve lesions, a greater bacillary load, and increasing antibody levels. The lesions in the chimpanzee and the sooty mangabey have been restricted predominately to the lepromatous pole (LL-BL) of leprosy (The Table).
MATERIALS AND METHODS
A group of 40 cynomolgus macaques were imported from The Philippines in 1990 to augment the breeding colony of the California Regional Primate Research Center (CRPRC) at the University of California, Davis. The animals were maintained in accordance with established standards of the U.S. Federal Animal Welfare Act, the American Association for Accreditation of Laboratory Animal Care (AAALAC) and the Guide for the Care and Use of Laboratory Animals (13). Animals were individually housed in stainless steel cages, and kept in climate-controlled rooms on a 12-hr dark/light cycle. They were fed a standard commercial diet containing 25% protein (Purina Monkey Chow 5045, pre-analyzed; Purina Mills Incorporated, Richmond, Indiana, U.S.A.) supplemented with fresh fruit and vegetables. Drinking water was provided ad libitum. Periodic pairing was carried out for breeding purposes. Tuberculin skin testing, as part of the routine preventive health program, was performed quarterly.
In January 1994, an 11-year-old, wild-caught, female cynomolgus macaque from this original shipment was brought to the attention of the veterinary service because of an equivocal tuberculin reaction and the presence of a skin rash. Previous clinical history included a crusty nasal discharge during the quarantine period and intermittent reports of epistaxis, crusty nares, and nasal discharge in late 1990 and early 1991. In March 1991, a physical exam revealed that the nasal philtrum was ulcerated and the nasal passages were partially closed with crusted material.
A follow-up comparative intradermal abdominal skin test was performed in January 1994 using mammalian tuberculin (Old Mammalian Tuberculin; Coopers Animal Health, Kansas City, Kansas, U.S.A.), avian tuberculin (Avian Old Tuberculin; National Veterinary Service Laboratory, Ames, Iowa, U.S.A.), and a sterile saline control. A small area of induration was noted at 24 and 48 hr only at the avian tuberculin inoculation site. The results, therefore, indicated an equivocal test for avian tuberculosis and a negative test for mammalian tuberculosis. Thoracic radiographs revealed no significant findings. Physical examination revealed generalized dry, scaly skin, depigmentation of hands and feet, and a missing nasal philtrum. Multiple ulcerated 2-3 mm diameter nodules were present symmetrically on the skin of the hands, wrists, ankles, feet, and tail base (Fig. 2). Lesions were more prominent on the dorsal surfaces of the hands and feet. Left axillary and bilateral inguinal lymphadenopathy were noted. Hematology revealed a mild normocytic normochromic anemia. Serum biochemistry results were within normal limits. The animal was negative for type D retrovirus by ELISA, Western blot, and virus isolation. Bacterial culture of a nasal swab resulted in growth of common commensal nasopharyngeal flora.
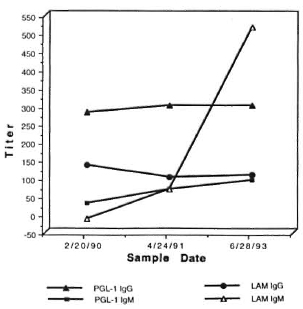
Fig. 1. Serology to PGL-I and LAM antigens
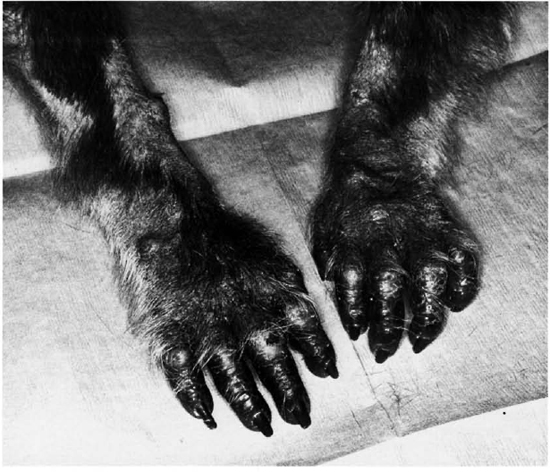
Fig. 2. Cynomolgus macaque with naturally acquired paucihacillary leprosy. Multiple ulcerated 2-3 nun di-ameter nodules were present asymmetrically in hand and wrist.
Selected cutaneous lesions were biopsied and specimens preserved either in 10% formalin or frozen in liquid nitrogen and stored at -70ºC. A broncho-alveolar lavage (BAL) was performed to obtain samples for cytology, culture, and analysis by polymerase chain reaction (PCR) for Mycobacterium DNA. The DNA segment target for amplification of the M. leprae genome used the 360-bp region, as described in detail elsewhere (32). This region encodes approximately 80% of the 18-kDa protein of M. leprae.
Longitudinal serum samples were analyzed by ELISA for IgG and IgM antibody levels to the phenolic glycolipid-I (PGL-I) and mycobacterium common lipoarabinomannan (LAM) antigens using techniques described previously (4, 5, 7, 9, 11). In early 1994, the animal was euthanized for diagnostic purposes and a necropsy was performed. A complete set of 10% neutral buffered formalin fixed tissues was sectioned and stained with hematoxylin and eosin (H&E) for examination. Selected tissues were stained using the Ziehl-Neelsen and Fite-Faraco techniques and examined for acid-fast bacilli (AFB).
RESULTS
Cytology of the cutaneous lesions revealed no ectoparasites or fungal elements. Impression smears revealed numerous AFB, mostly in packets, marked numbers of poly morphonuclear cells, a few with intracellular acid-fast organisms, and moderate numbers of mononuclear cells. Retrospective staining of the nasal smear demonstrated acid-fast organisms. BAL cytology was negative for AFB; however very little material was present on the slide. Mycobacterium DNA was detected at the genus level by PCR; however no reactivity was observed using M. tuberculosis or M. aviumintracellulare species typing probes (Roche Bioveterinary Services, Research Triangle Park, North Carolina, U.S.A.).
Tissue homogenates prepared from skin-biopsy specimens demonstrated AFB and positive reactivity for PCR amplified M. leprae (GWL Hansen's Disease Center, Carville, Louisiana, U.S.A.). Attempts to culture homogenate specimen by inoculation into the foot pads of mice were unsuccessful, but the quality of the inoculum was poor due to multiple freezing-and-thawing cycles.
Serum drawn at the time of the animal's arrival at CRPRC quarantine (February 1990) was strongly positive by ELISA for the IgG anti-PGL-I, which is specific for M. leprae. Retrospective analysis of stored sera demonstrated a significant rise in IgM anti-PGL-I and IgM LAM as early as June 1993, 6 months prior to detection of cutaneous lesions (Fig. 1). Single serum samples of four male breeders that had had direct contact with the affected animal were negative for till antibody tests.
Histopathology of biopsy specimens revealed a cutaneous infiltrate composed primarily of histiocytes with little epithelioid cell change or multinucleated cell formation. There were foci of necrosis, but these seemed associated with ulcers. Periodic Acid Schiff and Gomori methenamine silver stains failed to indicate the presence of fungal organisms
At necropsy significant gross pathological findings were limited to the nodular lesions on distal extremities and the necroulcerative lesion of the nares. Similar to the antemortem biopsy specimens, examination of these areas demonstrated granulomatous inflammation. However, in these samples, the inflammation included numerous epithelioid macrophages, including occasional multinucleated giant cells, scattered lymphocytes, and rare neutrophils (Fig. 3). Peripheral lymph nodes were distinguished by paracortical expansion, a drainage reaction which was mainly lymphohistiocytic, and sinus histiocytosis which frequently included multinuieated giant cells and took the form of multiple small granulomas. Of particular interest was the clear invasion of cutaneous nerves by histiocytes and multinucleated giant cells (Fig. 4). Ziehl-Neelsen and Fite-Faraco stains revealed very few AFB in the surface exudate, in the cutaneous histiocytic infiltrate, and within dermal nerves (Fig. 5). Immunohistochemical analyses were performed on sections of lesions from the nose and feet using antibodies directed toward lymphocyte markers CD3, CD4, and CD20, as well as the macrophage marker HAM56. Since CD3 is an antigen found on all T cells, the number of cells labelled by antibody to CD3 minus those labelled as CD4+ was used as an estimate of CD8+ T cells and, in this way, CD4 : CD8 ratios were calculated. These resuls indicate that T cells make up roughly 40% of the total mononuclear inflammatory cells (T cells, B cells, and macrophages) and that the CD4 : CD8 ratio in granulomatous lesions of skin, including those associated with nerves, is roughly 0.7. B cells marked by antibodies to CD20 are generally scattered at the periphery of granulomas while macrophages, marked by antibodies to HAM56, are distributed liberally throughout the affected sites.
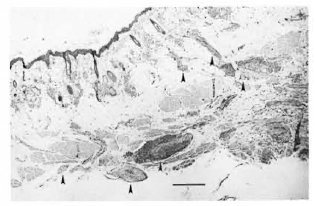
Fig. 3. Skin from the nose-multi focally in thedermis and subcutis, increased cellularity is mainly cen-tered on nerve fibers (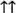 ) (H&E; scale bar = 100 µm).
) (H&E; scale bar = 100 µm).
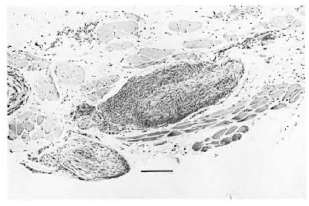
Fig. 4. Multifocally in the subcutis, two subcutic-ular nerve fibers contain an increased cellularity in theepineurium. In the larger nerve fiber, these cells extendcentrally, effacing the architecture. There is a diffusesuperficial dermal infiltrate, generally concentrated around adnexal structures, composed of an admixture of histiocytes and lymphocytes with scattered plasmacells and neutrophils (H&E; scale bar = 20 µm).
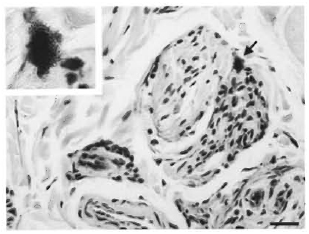
Fig. 5. Focally in the subcutis, a cell containingcytoplasmic, acid-fast positive material is present inthe epineurium. Subjacent to this cell, nerve fiber architecture is partially effaced by a disseminated moderate number of infiltrating histiocytes with lessernumbers of lymphocytes; the nerve fiber to the immediate left appears relatively uninvolved. INSERT: Higher magnification demonstrates acid-fast positivered coccobacilli within the cytoplasm of cell present inthe epineurium. (Ziehl-Neelsen; scale bar = 20 µm).
DISCUSSION
This is the first report of spontaneous leprosy in a member of the macaque species, the cynomolgus monkey (Macaca fascicularis). Clinical manifestation was characterized by cutaneous nodules on the extremities and AFB infiltration of the nasal mucosa, leading to chronic nasal discharge and epistaxis. The process extended to the nasal cartilage, leading to septal erosion and loss of the nasal philtrum.
The serologic data demonstrated an elevated IgG anti-PGL-I and the absence of significant IgM anti-PGL-I levels when the animal arrived at CRPRC, indicating a subclinical leprosy infection (7, 9, 11). Since leprosy is endemic in The Philippines, this animal could have made contact with a human or a nonhuman primate with leprosy in the source country. The prospect of acquiring the infection once in the United States is remote due to a very low potential of exposure coupled with leprosy's uniquely long incubation period, a minimum of 2-3 years and average of 3-5 years in humans. Data on natural transmission of leprosy among nonhuman primates is still limited, but the incubation period is also thought to be prolonged. A sooty mangabey developed multi-bacillary leprosy approximately 8 years after detection of the disease in its cagemate (8).
While leprosy patients constitute a source of contagion in humans, naturally infected animals or soil may also harbor the etiologic agent (21). Animal reservoirs of leprosy exist among feral nine-banded armadillos (Dasypus novemcinctus) (31) and, possibly, among nonhuman primates (8, 22-24). The occurrence of natural monkey-to-monkey transmission appears likely (8). Leprosy may be considered a zoonotic disease and transmission between armadillos and humans has been suggested (17). Because the incidence of naturally acquired leprosy in nonhuman primates is unknown, it is not possible to define their potential role in the epidemiology of the disease in humans. Nonhuman primates could serve as a reservoir in geographical areas where human leprosy is highly endemic, such as in The Philippines (30).
The ratio of anti-PGL-I IgM : IgG ELISA values in the animal reported here was <1 and, thus, is consistent with immunologic resistance and a cell-mediated immunity against M. leprae closer to the BB form of the disease for all three timepoints (10). These results are compatible with experimental data in the sooty mangabey, in which high IgG and low IgM levels to PGL-I were correlated with less severe disease and were of good prognostic value (7, 10). Longitudinally obtained serum samples from the cynomolgus macaque spanning several years permitted identification of preclinical leprosy retrospectively (Fig. 1 ). The data corroborate the fact that monitoring IgG and IgM levels to PGL-I and LAM may be useful in the recognition of preclinical leprosy, in confirming the diagnosis of leprosy, and in epidemiological studies of disease incubation and transmission (7, 10).
The final diagnosis of leprosy was confirmed by PCR analysis of skin-biopsy homogenates. This case clearly demonstrates that PCR amplification can be used specifically to detect small amounts of M. leprae DNA in skin-biopsy preparations, and it may be a useful clinical screening tool.
The lesions in naturally occurring leprosy of nonhuman primates, prior to this case, have been restricted predominately to the lepromatous pole (LL-BL) of the disease spectrum and contained diffuse infiltrates of macrophages and many AFB (The Table). In the case reported here, the cynomolgus macaque presumably exhibited a somewhat stronger immunologic response with lesions closer to the BB form of the disease. The lesions were more localized and contained variable numbers of lymphocytes with a mix of well- and poorly formed granulomas. Epithelioid macrophages as well as multinucleate giant cells were present, and the lesions contained few organisms.
Invasion of nerves by AFB and granulomatous inflammation is variable but distinct and constituted a defining characteristic of M. leprae lesions. The combination of the variably organized granulomatous inflammation, the paucibacillary nature of the lesions, the antibody responses to LAM and PGL-I, and the imnuinohistochemical data, place this animal in the paucibacillary BB intermediate category of leprosy lesions.
Intradermal tuberculin testing is the traditional method of screening nonhuman primate populations for mycobacterial infections. Exposure to nontuberculous mycobacteria, such as M. leprae, in some instances may sensitize primates to nonspecific mycobacterial antigens resulting in false-positive reactions, thus confounding tuberculin test results and compromising the preventive health program and surveillance of nonhuman primate colonies.
Experimental transmission studies attempted in cynomolgus macaques by Schöbl, el al. (1961) (28), Chaussinand (1941) (3) and Mcfazean and Ridley (1961) (9) were unsuccessful, or more recently partially successful (29). However, it has been demonstrated that African green monkeys (Cercopithecus aethiops) are susceptible to experimentally transmitted leprosy (2). These early failures may have been due to the use of an inappropriate inoculum, too few viable M. leprae, an inadequate route or regimen of inoculation, implantation of tissue fragments that stimulated host reaction or an insufficient period of observation post-inoculation to accommodate the long incubation period of this disease (18). Based on currently available information, susceptibility of cynomolgus macaques and other nonhuman primates to experimental infection with M. leprae should be re-evaluated.
This case widens the spectrum of naturally occurring M. leprae reported in nonhuman primates, suggesting that they might exhibit a spectrum of clinical and pathological forms of the disease similar to that seen in humans. Thus, nonhuman primates may provide invaluable animal models for the investigation of leprosy and its clinical disease.
Acknowledgment. The authors wish to thank Diana L. Williams from the Gillis W. Long Hansen's Disease Center, Carville, Louisiana, U.S.A., for kindly performing M. leprae PCR analysis and Gary Baskin for reviewing the histopathology samples. We also thank Abigail Spinner. Cindy Young and Philip Allen for their expert technical assistance. Pam Peterson provided instrumental editorial comments. This research was supported by the California Regional Primate Research Center NIH base grant RR00169.
REFERENCES
1. ALFORD, P. L., LIT., D. R., BINHAZIM, A. A., HUBBARD, G. B. and MATHERNE C. M. Naturally acquired leprosy in two wild-born chimpanzee. Lab. Anim. Sci. 46(1996)341-346.
2. BASKIN, G. B. Leprosy. In: Nonhuman Primates II. Jones, T. C., Mohr, U. and Hunt, R. D., eds. Berlin, Heidelberg: Springer-Verlag, 1993. pp. 8-14.
3. CHAUSSINAND, R. Contributions a l'étude de la lèpre. Inoculation du bacille de Hansen au singe. Int. J. Lepr. 9(1941)203-207.
4. CHO, S.-N., HUNTER, S. W., GELBER, R. IL, REA, T. II. and BKENNAN, P. J. Qualification of the phenolic glycolipid of Mycobacterium leprae and relevance to glycolipid antigenemia in leprosy. J. Infect. Dis. 153( 1986)560-569.
5. CHO. S.-N., SHIN, J. S., CHOI, I. H., KIM, D. I. and KIM, J. D. Detection of phenolic glycolipid I of Mycobacterium leprae and antibodies to the antigen in sera from leprosy patients and their contacts. Yonsei Med. J. 29(1988)219-224.
6. DONAHAM, K. J. and LEININGER, J. R. Spontaneous leprosy-like disease in a chimpanzee. J. Infect. Dis. 136(1977)132-136.
7. GORMUS, B. J., OHASHI, D. K., OHKAWA, S., WALSH, G. P., MEYERS, W. M., BRENNAN, P. J. and TRYGG, C. Serologic responses to Mycobacterium-leprae -speeilic phenolic glycolipid-I antigen in sooty mangabey monkeys with experimental leprosy. Int. J. Lepr. 56(1988)537-545.
8. GORMUS, B. J., WONF, R. H. BASKIN, G. B., OHKAWA, S., GERQN, P. J., WALSH, G. P., MEYERS, W. M., BlNFORD, C. H. and GREER, W. F. A second sooty mangabey monkey with naturally acquired leprosy: first reported possible monkey-tomonkey transmission. Int. J. Lepr. 56(1988)61-65.
9. GORMUS, B. J., XU, K., ALFORD, P. L., LEE, D. R., HUBBARD, G. B., EICHBERG, J. W. and MEYERS, W. M. A serologic study of naturally acquired leprosy in chimpanzees. Int. J. Lepr. 59( 1991)61-65.
10. GORMUS, B, J., XU, K., CHO, S.-N., BASKIN, G. B., BOHM, R. P., MARTIN, L. N., BLANCHARD, J. L., MACK, P. A., RATTERREE, M. S., MEYERS, W. M. and WALSH, G. P. Expérimental leprosy in monkeys. II. Longitudinal serological observation in sooty mangabey monkeys. Lepr. Rev. 66(1995)105-125.
11. GORMUS, B. J., XU, K., MEYERS, W. M., WALSH, G. P., LEVIS, W. R. and MEEKER, H. C. Antibodies to lipoarabinomannam antigen in sooty mangabey monkeys experimentally inoculated with Mycobacterium leprae. Int. J. Lepr. 58(1990)65-72.
12. HUBBARD, G. B., LEI:, D. R., EICHBERG, J. W., GORMUS, B. J., XU, K. and MEYERS, W. M. Spontaneous leprosy in a chimpanzee ( Pan troglodytes). Vet Pathol. 28(1991)546-548.
13. INSTITUTE FOR LABORATORY RESOURCES. Guide for the care and use of laboratory animals. Washington, D.C.: National Academy Press, 1996.
14. KAPLAN, G. and COHN, Z., A. Leprosy and cell mediated immunity. Curr. Opin. Immunol. 3(1991)91-96.
15. LEININGER, J. R., DONAH AM, K. J. and MEYERS, W. M. Leprosy in a chimpanzee; postmortem lesions. Int. J. Lepr. 48(1980)414-421.
16. LEININGER, J. R., DONAHAM, K. J. and RUBINO, M. J. Leprosy in a chimpanzee; morphology of the skin lesions and characterization of the organism. Vet. Pathol. 15(1978)339-346.
17. LUMPKIN, L. R., COX, G. F. and WON . J. E. Leprosy in live armadillo handlers. J. Am. Acad. Dermatol. 9(1983)899-903.
18. MARTIN, L. N., GORMUS, B. J. and WOLF, R. H. Experimental leprosy in nonhuman primates. Adv. Vet. Sei. Comp. Med. 28(1984)201-236.
19. MCFAZEAN, J. A. and RIDLEY, D. S. Studies on the inoculation of Mycobacterium leprae into monkeys. Trans. R. Soc. Trop. Med. Hyg. 55(1961)235-238.
20. MEHRA, V. and MODLIN, R. L. T-lymphoeytes in leprosy lesions. Curr. Top. Microbiol. Immunol. 155(1989)97-109.
21. MEYERS, W. M., GORMUS, B. J. and WALSH, G. P. Nonhuman sources of leprosy. Int. J. Lepr. 60(1992)477-481.
22. MEYERS, W. M., GORMUS, B. J., WALSH, G. P., BASKIN, G. B. and HUBBARD, G. B. Naturally acquired and experimental leprosy in nonhuman primates. Am. J. Trop. Med. Hyg. 44(1991)24-27.
23. MEYERS, W. M., WALSH, G. P., BROWN, H. L., BINFORD, C. H., GERONI, P. J., WOLF, R. H., GORMUS, B. J. and MARTIN, L. Leprosy in the mangabey monkey( Cercocebus torquatus atys, "sooty mangabey"). Int. J. Lepr. 49(1981)500-502.
24. MEYERS, W. M., WALSH, G. P., BROWN, H. L., BINFORD, C. H., IMES, G. D. JR., HADFIELD, T. L., SCHLAGEL, C. J., FUKUNISHI, Y., GLRONE, P. J., WOLF, R. H., GORMUS, B. J., MARTIN, L. N., HARBOE, M. and IMAEDA, T. Leprosy in a mangabey monkey-naturally acquired infection. Int. J. Lepr. 53(1985)1-14.
25. MLLLER, R. A. Infectious diseases. In: Harrison's Principles of Internal Medicine. 13th edn. New York: McGraw-Hill, Inc., 1994, pp. 718-722.
26. REES, R. J. W. and MCDOUGALL, C. Airborne infection with Mycobacterium leprae in mice. J. Med. Microbiol. 10(1977)63-68.
27. RIDLEY, D. S. and JOPLING, W. H. Classification of leprosy according to immunity; a live-group system. Int. J. Lepr. 34(1966)255-273.
28. SCHORL, O., PINEDA, E. V. and MRYAO, I. Clinical skin lesions in Philippine monkeys resulting from experimental inoculation with human leprous material. Philipp. J. Sei. 41(1930)233-243.
29. WALSH, G. P., DELA CRUZ, E. C. ABALOS. R. M., FAJARDO, T. T, GUTDO, L. S., CELLONI. R. V, MEYERS, W. M., GORMUS, B. J., RESUELLO, R. and LANGE, F. W. Leprosy studies in Philippine cynomolgus monkeys ( Macaco fascicularis): preliminary results.(Abstract). Int. J. Lepr. 58(1990)193.
30. WALSH. G. P., MEYKRS, W. M. and BINFORD, C. H. Leprosy as a zoonosis: an update. Acta Leprol. 6(1988)51-60.
31. WALSH, G. P., STORRS, E. E., BURCHFIELD, H. P., COTTRELL, E. H., VLDRINE, M. F. and BLNFORD, C. H. Leprosy-like disease occurring naturally in armadillos. J. Reticuloendothel. Soc. 18(1975)347-351.
32. WILLIAMS, D. L., GILLIS, T. P., BOOTH, R. J. and WATSON, J. D. The use of A specific DNA probe and polymerase chain reaction for the detection OF Mycobacterium leprae. J. Infect. Dis. 162(1990)193-200.
1 C . R. Valverde. D.V.M., California Regional Primate Research Center, University of California-Davis, One Shields Avenue. Davis, California 95616-8542, U.S.A.
2 D. Canfield, D.V.M., California Regional Primate Research Center, University of California-Davis, One Shields Avenue. Davis, California 95616-8542, U.S.A.
3 R. Tarara, D.V.M., Ph.D., California Regional Primate Research Center, University of California-Davis, One Shields Avenue. Davis, California 95616-8542, U.S.A.
4 M. I. Lsteves, D.V.M., Division of Comparative Medicine. Massachusetts Institute of Technology, Cambridge. Massachusetts, U.S.A.
5 B. J. Gormus. Ph.D., Department of Microbiology, Pathology and Veterinary Services, Tulane Regional Primate Research Center, Covington, Louisiana. U.S.A.
Reprint requests to Dr. Valverde at the above address or fax: 530-752-2880; email: crvalverde@ucdavis.edu
Received for publication on 17 March 1998.
Accepted for publication in revised form on 4 May 1998.