- Volume 66 , Number 2
- Page: 159–69
The occurrence of reactions and impairments in leprosy: experience in the Leprosy Control Program of three Provinces in Northeastern Thailand, 1978-1995. II. Reactions
ABSTRACT
Aim: This is the second paper in a series of three papers on the occurrence of reactions and impairments in leprosy in Thailand, and focuses on the prevalence and incidence of reactions, including silent neuropathy. Study design: A population-based, prospective cohort study. Study population: All 640 newly diagnosed and registered leprosy patients in three neighboring provinces in northeastern Thailand registered between October 1987 and September 1990 were included [420 paucibacillary (PB) and 220 multibacillary (MB)]. This group was followed up (actively and passively) until the end of 1995. Methods: Clinical data and data on the sensibility and motor function of the eyes, hands and feet were obtained when appropriate. The occurrence of reactions, including silent neuropathy, at the beginning of, during and after treatment was ascertained. During surveillance mild late reactions were also recorded. Results: Severe reversal reactions (RR) at the start of and during treatment were seen in 2.6% [confidence interval (CI) 1.1 4.1 ] of the PB and 29% (CI 23-35) of the MB patients. In the PB group the majority (82%) of severe RR were found at the start of treatment. In the MB group 35% of the severe RR were found at the start of treatment and another 59% during the first year of treatment. It is shown that there is a statistically highly significant increasing proportion of patients with severe RR starting f rom tuberculoid and going toward borderline lepromatous. The incidence rate of severe RR during treatment was 1.4 (CI 0.464.5) per 100 person-years at risk (PYAR) for PB patients and 12 (CI 9.0-16) per 100 PYAR for MB patients. Late (mild and severe) RR were seen in 2.7% of the PB and 9% of the MB patients (35% of these reactions being considered severe). Late reactions were mainly seen in borderline tuberculoid (PB group) and in borderline lepromatous patients. Recent silent neuropathies were seen at first examination and during treatment in 1.4% of the PB and 4.1% of the MB patients. During surveillance only a few silent neuropathies were seen. If all severe RR, severe erythema nodosum leprosum and silent neuropathies at the start of, during and after treatment were added together, then 53% of the borderline lepromatous and 42% of the lepromatous patients had or developed one or another serious complication in need of steroid treatment.RÉSUMÉ
But: Cet article est le second d'une série de trois traitant de l'apparition d'états rédactionnels et d'infirimités hansénienne en Thaïlande, et expose en particulier la prévalence et l'incidence des états réactionnefs, incluant les neuropathies "silencieuses". Type d'étude: Etude prospective par cohortes, basée sur une population. Population d'étude: La totalité des 640 nouveaux cas de lèpre |420 paucibaeillaires (PB) et 220 multibacillaires (MB)] diagnostiqués dans les 3 provinces conligues de la Thaïlande du nord-est, enregistrés de octobre 1987 à septembre 1990, l'ut passivement et activement suivie jusqu'à lin 1995. Méthode: Les données cliniques et les données, lorsque jugées appropriées, concernant les functions sensitives et motrices des yeux, des mains et des pieds furent obtenues. L'apparition de réactions, y compris les neuropathies silencieuses, fut déterminée au début, au cours et après le traitement. Durant le suivi, les états réactionnels légers tardifs ont également été enregistrés. Results: De sévères réactions de réversion (RR) au début et durant le traitement ont été observées chez 2,67c [intervalle de confiance (IC) 1,1-4,4] des cas PB et 29% (IC: 23-35) des patients MB. Chez le groupe PB, la majorité (827) des RR sévères fut découverte au début du traitement. Parmi le groupe MB, 357' des RR sévères a été vu en début de traitement et 597- additionnel durant la première année de traitement. L'étude a permis de montrer qu'il y avait une proportion croissante statistiquement hautement significative de patients avec des RR sévères, débutant chez les cas tuberculides et s'accroissant jusqu'aux cas lépromateux borderlines. Le taux d'incidence des RR sévères au cours de traitement était de 1,4 (IC: 0,45-4,5) pour 100 personnes-années à risque (PAAR) chez les patients PB et de 12 (IC: 9,0-16) pour 100 PAAR chez les patients MB. Les RR tardives (légères et sévères) ont été veus chez 2,77- des cas PB et 97- des patients MB (35% des ces réactioncs étant considérées sévères). Les réactions tardives furent observées principalement chez les cas tuberculides borderlines (groupe PB) et les patients lépromateux borderlines. Des neuropathies silencieuses récentes furent détectées au cours du premier examen et durant le traitement chez 1,4% des patients PB et 4,17' des patients MB. Au cours du suivi post traitement, il n'a été vu que quelques neuropathies silencieuses. Si tous les cas de RR sévères, d'érythèmes noueux lépreux sévères et de neuropathies silencieuses en début, au cours et après le traitement sont additionnés ensemble, alors 53% des patients lépromateux borderlines et 42% des patients lépromateux avaient déjà ou ont développés l'une ou l'autre de ces complications sérieuses nécessitant un traitement aux corticostéroïdesRESUMEN
Objetivo: F.ste es el segundo de tres artículos sobre la oceurrencia de reacciones en la lepra y el empeoramiento consecuente de los pacientes de Tailandia. Se incluyen las neuropatías subclínicas. Diseño del estudio: Se trata de un estudio poblacional prospectivo de cohortes. Población de estudio: Los 640 casos nuevos de pacientes con lepra [420 paucibacilares (PB) y 220 multibacilares (MB)] diagnosticados en tres provincias de Tailandia entre Octubre de 1987 y Septiembre de 1990. El seguimiento de los pacientes se continuó hasta finales de 1995. Métodos: Además de los datos clínicos se utilizaron técnicas para medir la sensibilidad y la función motora de los ojos, los pics y las manos. Se buscaron y registraron las evidencias de reacción leprosa (incluyendo las neuropatías subclínicas) al comienzo, durante y después del tratamiento. Durante el seguimiento también se registraron las reacciones tardías moderadas. Resultados: Se observaron reacciones reversas severas (RR) al comienzo y durante el tratamiento, en el 2.6% (intervalo de confianza, CI = 1.1 -4.1 ) de los pacientes PB y en el 29% (CI 23-25) de los pacientes MB. En el grupo PB la mayoría (82%) de las RR severas se encontraron al inicio del tratamiento. Fn el grupo MB un 35% de las RR severas se observaron al inicio del tratamiento y otro 59% durante el primer año de tratamiento. Se encontró que la proporción de pacientes con RR severa aumentó significativamente del extremo tuberculoide al extremo lepromatoso subpolar. La tasa de incidencia de RR severas durante el tratamiento fue de 1.4 (CI 0.46-4.5) por 100 personaaños en riesgo (PYAR) para los pacientes PB y de 12 (CI 9.0-16) por 100 PYAR para los pacientes MB. Se observaron RR tardías (moderadas y severas) en el 2.7% de los pacientes PB y en el 9% de los pacientes MB (siendo severas el 35% de estas reacciones). Las reacciones tardías se observaron principalmente en el grupo tuberculoide subpolar (grupo PB) y en los pacientes con lepra lepromatosa subpolar. Las neuropatías subclínicas recientes se observaron en el primer examen y durante el tratamiento, en el 1.4% de los pacientes PB y en el 4.1% de los MB. Durante el seguimiento sólo se descubrieron algunas neuropatías subclínicas. Sumando todas las RR severas con los casos de eritema nodoso leproso y con las neuropatías subclínicas al inicio, durante y después del tratamiento, el 53% de los pacientes con lepra lepromatosa subpolar y el 42% de los pacientes lepromatosos habrían desarrollado alguna complicación seria cuyo tratamiento ameritaría ul uso de csteroides.A major problem in leprosy is the development of immunological reactions and, in particular, the loss of peripheral nerve function which usually develops during reactions. Type 1 reaction is one of the major causes of nerve damage in leprosy (13, 23) . The other type of lepra reaction is called type 2 reaction or erythema nodosum leprosum (ENL). These reactions and the potential loss of nerve function can occur at any time in the disease process, before the start of treatment, during treatment and even after treatment. It has been proposed to report silent neuropathies separately from reversal reactions because of their uncertain etiology (28).
This is the second paper in a series of three papers and focuses on the prevalence and incidence of reactions and of silent neuropathy in Thailand. The other two papers report on: I. General Overview of the Study (some epidemiological observations, delay in detection, MDT completion rates, relapses); III. Neural and Other Impairments.
PATIENTS AND METHODS
A total of 640 newly diagnosed leprosy patients [420 paucibacillary (PB) and 220 multibacillary (MB) patients] from three provinces (Mahasarakham, Roi-et and Kalasin) in northeastern Thailand were enrolled in this study. The study lasted from October 1987 until the end of 1995. (For an overview of the study see Part I.)
The leprosy control staff were requested to refer new patients for confirmation of diagnosis and classification and, in case of problems or complications, to monthly referral clinics at the provincial health offices. At the time of release from multidrug therapy (MDT) patients were advised about the importance of reporting to the service as soon as any problem arose.
Routinely, impairment records (32) were made on registration then every 3 months. In case of complications recordings were made monthly. Patients under surveillance were examined annually. The leprosy field workers were allowed to start prednisolone in patients with early signs of nerve function impairment (NFI) or other signs of severe leprosy reaction, but were expected to refer such patients to the monthly referral clinics.
Reversal reactions (RR) and erythema nodosum leprosum (ENL) reactions were differentiated into mild and severe reactions. In this study, only severe reactions at the start of and while on treatment were recorded. However, after release from treatment (RFT) both mild and severe reactions were taken into account to differentiate late reaction from relapse. The definition of a severe RR (not including silent neuropathy) and the indications for the use of prednisolone were similar to those of Becx-Bleumink, el al. (3) and Rose and Waters (25). Edema of the face, hands or feet in conjunction with an inflamed lesion was considered an additional sign of a severe reaction. Patients with signs of acute neuritis (pain or tenderness) and signs of recent NFI were considered to have a severe reaction. Because of its uncertain etiology, silent neuropathy (28, 29) was not included with the severe reversal reactions. Recent (less than 6 months) silent neuropathy was defined as sensory or motor impairment without nerve pain, nerve tenderness, paresthesia or numbness and without signs of a skin reaction (28). If any of the signs of a severe RR or silent neuropathy were present, a semi-standardized prednisolone regimen (initial dose of 40 mg, 35 mg and/or 30 mg each daily for 2 weeks, followed by 25 mg daily for 4 weeks, then tapering off by 5 mg every month; total duration of 5 to 6 months on the average) was prescribed (17, 18). In those cases where no clear improvement was seen (or more improvement was hoped for) a maintenance dose of 15 mg to 20 mg a day was continued for an additional month or more. The population of northeast Thailand is, in general, small in stature; adults often weighing less than 50 kilograms.
The definition of a severe ENL reaction was similar to that of Becx-Bleumink, et al. (3). In the case of a severe ENL reaction a prednisolone course of short duration (6 weeks) was prescribed. At the same time the dose of clofazimine was increased to 200 mg or 300 mg daily in patients with repeated severe ENL. This high dosage of clofazimine was given for at least 3 months before tapering off in the absence of a new ENL reaction and after prednisolone had been discontinued. Thalidomide was not available in Thailand. In the case of ENL with signs of neuritis and iridocyclitis, the dose of prednisolone and the duration of the course depended upon clinical signs and nerve function test findings. Lepromatous patients with signs of acute neuritis and recent nerve function impairment but without signs of ENL were treated (and classified) as suffering from a severe RR.
Signs of a late RR can range from a mild reaction (previous lesions reappearing or existing lesions becoming more clearly visible) to severe reaction involving skin and/or nerves. Late ENL reactions can range from mild to severe ENL. A late reaction had to be differentiated from a relapse (1). Mild reactions received prednisolone in order to differentiate late reaction from relapse. Patients with a late reaction received prednisolone without a temporary cover of World Health Organization MDT (WHO/MDT).
History taking; inspection of the eyes, hands and feet; palpation of the nerves; voluntary muscle testing (VMT) and sensory testing (ST) provided the relevant information needed for the early diagnosis of NFI. As a sensory test, the ballpoint pen test (32) was used (hand: 10 sites; foot: 12 sites). The assessment of the motor function was done on a three-scale VMT scale (strong, weak, paralyzed). A patient was considered to have NFI if there was a deterioration in the VMT score or 2 points or more in the ST score compared to the previous result. NFI of less than 6-months' duration was recorded as "recent" (29). In this study, the WHO disability three-grade system was followed with a few exceptions (32).
Statistical methods. When determining the difference between two unpaired observations in the case of large samples the normal test was used (12). The chi-squared test was used to analyze the difference between proportions and for trends (Epi Info software, version 6). Incidence rates were calculated as the number of patients developing a severe reaction during the follow-up period divided by the cumulative person-years at risk (PYAR). Patients lost to follow up or who had died or transferred out only contributed to the denominator as long as they were followed up. If patients already had a severe reaction the time from the start of treatment to the second severe reaction was counted. They only contributed to the denominator until the time of the start of the second reaction. A p value of less than 0.05 was used as the level of statistical significance. Confidence intervals (95%) are given for the prevalence and incidence rates.
RESULTS
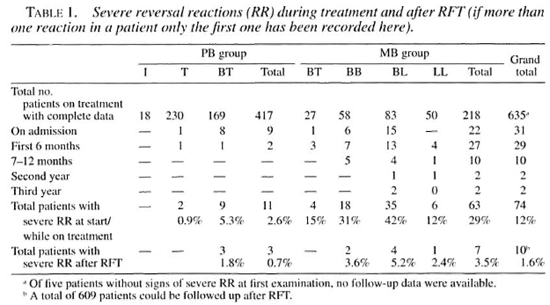
Eleven PB (2.6%, 95% CI 1.1-4.1) and 63 MB patients (29%, CI 23-35) had signs of a severe RR either at the start of treatment or developed one during treatment (Table 1). If a patient was suffering from more than one episode of severe RR only the first one was counted here. Eleven patients (1 BT, 3 BB and 7 BL) (15% of the patients with severe RR) developed more than one episode of severe RR. Six of these 11 patients developed a new episode of severe RR within 3 months of finishing the prednisolone course for the previous severe RR. There were no clear differences between patients with repeated RR and those with only one episode of severe RR. One BL and two LL patients developed both a severe RR and a severe ENL reaction while on treatment (although at different times). No statistically significant difference was found in the occurrence of reactions between males and females in the PB and MB groups.
In the PB passive case-finding group 4% of the patients had or developed a severe RR; in the active case-finding group this was 1% which is statistically not significant. In the MB passive case-finding group 31 % of the patients had or developed severe RR. In the active case-finding group it was 25%, which is also statistically not significant. Patients with a longer delay in detection were not more likely to develop a severe RR than patients with a shorter delay in detection. Particularly in the MB group, severe RR were equally present in the different delay groups (data not shown).
In the PB group the majority (82%) of severe RR were found at the first examination. The prevalence of severe RR in the PB group at first examination was 2.2% (95% CI 0.8-3.6). In the MB group 35% of the severe RR were found at the start of treatment, an additional 43% during the first 6 months of treatment (start and first 6 months total 78%), and another 16% during the second half of the first year of treatment (start and first year total 94%). The prevalence of severe RR in the MB group at first examination was 10.1% (CI 6.1-14.1).
In the PB group more BT patients and in the MB group more BL patients developed severe RR. There was an increasing proportion (highly significant) of patients with severe RR (at first examination and during treatment) from those at the TT pole of the leprosy spectrum toward those with BL (TT 1%; BT 5%; BT/MB 15%; BB 31%); BL 42%; chi-squared for linear trend: 121.390 with p value of 0.000001). Also, when late severe RR and silent neuropathies were included this trend stayed highly significant.
Incidence rate of severe RR during treatment. Three PB patients developed severe RR during treatment (one of these patients also had a reaction at first examination). This translates as an incidence rate for severe RR of 1.4 (CI 0.46-4.5) per 100 PYAR during WHO/MDT PB (given that the treatment lasted for 6 months per PB patient). Forty-six MB patients developed severe RR during the first 24 months of treatment. This translates as an incidence rate for severe RR of 12 (CI 9.0-16) per 100 PYAR during the first 24 months of WHO/MDT MB.
Association between WHO disability grading at the start of treatment and severe RR. It was found that patients with WHO disability grades 1 and 2 at the start of treatment were more likely for both the PB and the MB groups to have severe RR at diagnosis (Table 2). This was statistically significant. No such association was found between impairments at start of treatment and the risk of developing severe RR during treatment.
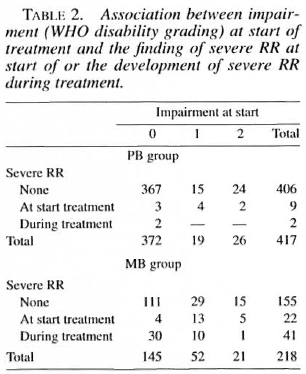
Silent neuropathy during treatment. Six PB patients (1.4%) and nine MB patients (4.1%) were diagnosed with a recent silent neuropathy at first examination or developed one during treatment (Table 3). One MB patient was diagnosed with an old (duration of more than 6 months before being diagnosed) silent neuropathy. The majority of the silent neuropathies were seen at the start of treatment. Fourteen of the 15 patients with a recent silent neuropathy received steroid treatment (similar regimen as severe RR).
Prevalence (rate) at start and incidence rate during treatment of severe RR and silent neuropathies counted together. The prevalence of severe RR plus (recent) silent neuropathy at the start of treatment in PB patients was 14 (3.5%, CI 1.8-5.2); in MB patients 27 (12.3%, CI 7.9-16.7). The incidence rate for severe RR plus (recent) neuropathy during the first 6 months of WHO/MDT PB was 1.9 per 100 PYAR; during the first 24 months of WHO/MDT MB, 14 per 100 PYAR.
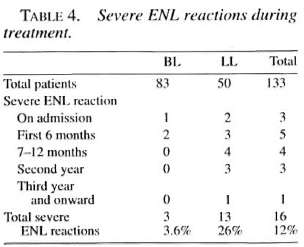
Severe ENL reactions during treatment. Three BL patients (3.6%) and 13 LL patients (26%) suffered from severe ENL at the first examination or during treatment (Table 4). Only the first episode of a severe ENL reaction has been counted here. Of these, seven patients (44%) suffered from repeated (more than two times) attacks of severe ENL.
Late reactions. Eleven PB patients (2.7%, CI 1.1-4.3) and 18 MB patients (9%, CI 5-13) were seen with late RR (Table 5). Ten of the 29 episodes were considered severe and 19 were classified as mild late RR. Most late reactions in the PB group were seen in BT patients; in the MB group, in the BL patients.
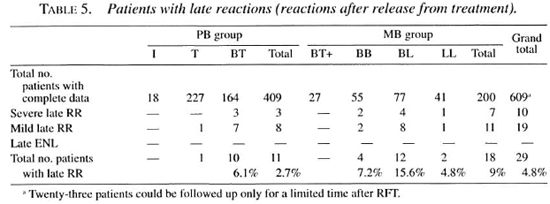
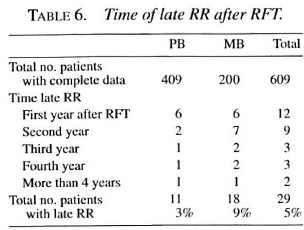
The majority (72%) of the late RR appeared during the first 2 years after RFT (Table 6). One PB patient developed signs of a late reaction 4.5 years after RFT. One MB patient developed signs of a late reaction almost 5 years after RFT. One PB patient developed a late reaction twice: the first during the second year after RFT, the second 5.5 years after RFT.
Late silent neuropathy. Five patients out of 609 were diagnosed with a late silent neuropathy (3 PB and 2 MB; 3 the first year, 1 the second year, and 1 the third year after RFT). Four of them did not receive prednisolone: in two patients the new NFI was recorded, but no action was taken; in the other two patients the silent neuropathy was only recognized more than 6 months after the start of the NFI (therefore the damage was considered to be old).
Severe RR, severe ENL and silent neuropathy during and after treatment. One BT and two BL patients suffered from severe RR during treatment and during surveillance. One BL and two LL patients suffered from both ENL and severe RR. Adding the occurrences of severe RR, severe ENL and silent neuropathy at the start of, during and after treatment: 53% of the BL patients (35 severe RR plus 4 silent neuropathies plus 2 severe ENL plus 2 late severe RR plus 1 late silent neuropathy = 44 out of 83) and 42% of the LL patients (6 + 3 + 11 + 1, respectively = 21 out of 50) developed one or another serious complication (in need of prednisolone).
DISCUSSION
Several authors have given an overview of case definitions and estimates of the frequency of reversal (type 1) reactions in leprosy (2, 13, 30). Because of the widely different case definitions, it is difficult to compare reported frequencies of RR in PB and MB patients in different studies (Table 7). The evident lack of a succinct and comprehensive definition of a leprosy reaction and of neuritis (as a possible manifestation of such a reaction) remains a problem (9).
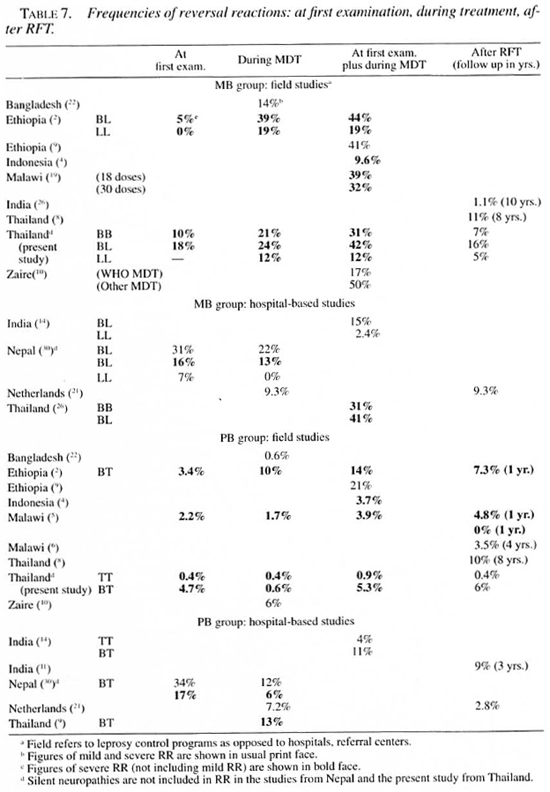
Reported frequencies (at first examination plus during treatment) of severe RR in BL patients ranged from 29% (Nepal) to 44% (Ethiopia); in BT patients, from 5.3 (present study) to 23% (Nepal) (Table 7). The PB patients in our study had significantly less severe RR at the start of/during treatment compared to the MB patients (Table 1). The same has been reported from most other studies (Table 7).
Severe RR at start of and during treatment. It is a common finding that most severe RR are found either at the start of or during the first year of treatment (2, 9, 30). The period of greatest risk for RR among patients not in reaction at the time of diagnosis is the first 6 to 12 months of treatment (13). "It seems that within the first 6 to 9 months of treatment, effective chemotherapy, together with the body's ability to dispose of M. leprae antigens, diminishes the amount of M. leprae antigens to a level below the 'threshold' needed to give rise to a damaging reversal reaction" (17).
In this study the majority of reactions in PB patients were found at first examination; most MB patients developed such a reaction after the start of treatment. Spontaneous RR are a feature of borderline disease, and are not necessarily a result of antileprosy treatment (17). However, there appears to be a clear association between the initiation of treatment and the development of reactions in MB patients (14, 17).
It has been shown that there is a statistically significant increasing proportion of patients with severe RR, starting from TT and going toward BL. It has already been stated that "the risk of developing a RR for patients with 'extensive disease' (3 or more body areas involved) was 10 times that of patients with limited disease" (30).
Incidence of RR. The incidence of RR is significantly higher among BL patients; similarly, the incidence of ENL reactions is significantly higher in LL patients (2).
In Nepal, the overall incidence rates of RR (mild and severe) in patients with limited disease was approximately 1/100 PYAR; among patients with three or more body areas involved, 12/100 PYAR. Patients had been followed up, on average, 20.7 months at the time of data analysis (30). These data compare well with the data in this study. In Malawi, the overall incidence rate of marked (needing prednisolone) RR was 3 per 100 PYAR years in the PB group during WHO/MDT (6). In Bangladesh, the incidence rates of RR in PB and MB patients during MDT were 1 and 6 per 100 PYAR (22). The study in Bangladesh was retrospective and a clear definition of a RR was not given.
Recurrent episodes of RR. From the studies of Naafs, et al. (15-18) it became clear that if prednisolone was given for too short a period, the likelihood of a rebound reaction and NFI was greatly increased, especially in MB patients (17). Corticosteroids both decrease the inflammatory processes (with reduction of intraneural edema and decreasing compression) and suppress cell-mediated immunity. When given over a longer period, they will prevent the recurrence of the reaction and may improve nerve recovery (17). In 10 MB patients (16%) in this study a recurrent episode of severe RR during treatment was diagnosed. However, almost half of those recurrent reactions happened within 3 months after stopping the prednisolone course of the previous reaction. Should those (within 3 months recurrent) reactions be considered as a flare up of the previous reaction and not as a new reaction? Was the duration of the prednisolone treatment in these patients insufficient? Theoretically, immunosuppressive treatment should continue throughout the period during which the antigenic load is sufficient to trigger the cell-mediated immune response. Patients with tuberculoid leprosy may require treatment for 3-6 months, midborderiine for 6-9 months, and borderline lepromatous patients even for as long as 18-24 months (15, 27). In the hospital-based studies (Table 8) many patients experienced recurrent episodes of severe RR. Was the immunosuppression in some of these patients of too short a duration or do hospitals tend to get more severely affected patients? In India patients with recent paralytic deformities received high doses of prednisolone for long periods of time. Their treatment outcome was comparable with other studies and in only 4% of the patients was a recurrent reaction diagnosed (27). Recurrent reactions can lead to additional impairments. In general, recurrent episodes of reactions are more common as ENL than as RR (26).
Risk factors for RR. Several risk factors for the occurrence of RR in leprosy have been cited, for example, BCG vaccination, pregnancy and the puerperium and chemotherapy (13). Laboratory markers that identity patients with an increased risk for RR have been studied (24). In this study, no relationship was found between having impairments at the start of treatment and the risk of developing severe reactions during treatment; however, a statistically significant relationship was found between having impairments at the start of treatment and having a reaction at the same time. In Malawi, more RR were diagnosed in PB patients after RFT (especially during the first year) than during treatment, and the RR risk after treatment among self-reporting patients was found to be higher than among active case finding (5). This could not be confirmed in this study. In Nepal it was found that the most significant risk factor for RR was the extent of the disease measured by a count of body areas with clinical signs of leprosy (30).
Silent neuropaththy. In most published studies the silent neuropathies are included with the severe RR (Table 7). In Ethiopia in a study of reaction and neuritis, 35 out of 53 (66%) cases of "neuritis" were considered to be cases of "silent neuritis" (9). In Indonesia one third of all reaction patients suffered from "silent neuritis" (4). In this study 14% of all severe reaction cases suffered from a recent silent neuropathy (if recent silent neuropathies are included with severe RR and ENL).
van Brakel and Khawas proposed reporting silent neuropathies separately from RR because of their uncertain etiology (28). In Nepal, 6.8% of all new patients were found with a silent neuropathy at the first examination. Silent neuropathy during and after treatment was found in 6.4% of the Nepal patients (28) and in 1.8% of the patients in this study. An explanation for this difference cannot be given.
Late reactions. In the PB group more BT patients and in the MB group more BL patients developed a late reaction. The proportion of patients with a late reaction could have been higher if all MB patients had been treated for a fixed duration of 24 months only. In this study no patients were seen with a late ENL reaction since BL and LL patients were treated until skin-smear negative.
In this study, 2.7% of the PB patients developed a late RR. In Malawi, 3.5% of the PB patients developed a late reaction during the first 4 years of follow up; 88% of those reactions were seen the first year after RFT (6). In India, 9% of the PB patients in the WHO-rccommended regimen group developed a late reaction during the first year after stopping treatment, but none in the group for whom dapsone was continued for another 6 months after WHO/MDT (11, 16). In Ethiopia, 8% of the BT patients developed a late reaction the first year after stopping treatment (2). Figures of late reactions in MB patients are more difficult to come by (Table 7).
In this study, 9% of the MB patients developed a late RR. In a previous study from the same area of Thailand late RR were seen in 11 % of the MB patients using WHO/MDT fixed duration treatment of 2 years (8). In India, in a cohort of MB patients (treated with MDT for 2 years or longer until skin-smear negative) only 1.1% experienced RR during almost 10 years of surveillance (31). Late RR happen mostly within the first 3-4 years after RFT (2). Clinicians should be aware that very late reactions may occur, and in India the longest treatment-reaction interval was 6.5 years (14).
REFERENCES
1. BECX-BLEUMINK, M. Relapses among leprosy patients treated with multidrug therapy: experience in the Leprosy Control Program of the All Africa Leprosy and Rehabilitation Training Center (ALERT) in Ethiopia: practical difficulties with diagnosing relapses; operational procedures and criteria for diagnosing relapses. Int. J. Lepr. 60(1992)421-435.
2. BECX-BLEUMINK, M. and BERHE, D. Occurrence of reactions, their diagnosis and management in leprosy patients treated with multidrug therapy: experience in the Leprosy Control Program of the All Africa Leprosy and Rehabilitation Training Center (ALERT) in Ethiopia. Int. J. Lepr. 60(1992)173-184.
3. BECX-BLEUMINK, M., BERHE, D. and 'T. MANNETJE, W. The management of nerve damage in the leprosy control services. Lepr. Rev. 61(1990)1-11.
4. BERNINK, E. H. M. and VOSKKNS, J. E. Study on the detection of leprosy reactions and the effect of prednisone on various nerves, Indonesia. Lepr. Rev. 68(1997)225-232.
5. BOERRIOTER, G., PONNIGHAUS, J. M. and FINE:, P. E. M. Preliminary appraisal of a WHO-recommended multiple drug regimen in paucibacillary leprosy patients in Malawi. Int. J. Lepr. 56(1988)408-417.
6. BOERRIOTER, G., PONNIGHAUS, J. M., FINE, P. E. M. and WILSON, R. J. Four-years follow-up results of a WHO-recommended multiple-drug regimen in paucibacillary leprosy patients in Malawi. Int. J. Lepr. 59(1991)255-261.
7. BWIRE, R. and KAWUMA, H. J. S. Hospital-based epidemiological study of reactions, Buluba Hospital, 1985-89. Lepr. Rev. 64(1993)325-329.
8. DASANANJALI, K., SCHREUDER, P. A. M. and PIRAVAVARAPORN, C. A study of the effectiveness and safety of the WHO MDT regimen in the northeast of Thailand, a prospective study, 19841996. Int. J. Lepr. 65(1997)28-36.
9. DE RIJK, A., GABRE, S., BYASS, P. and BERHANU, T. Field evaluation of WHO MDT fixed duration at ALERT, Ethiopia: the AMFES project-II. Reaction and neuritis during and after MDT in PB and MB leprosy. Lepr. Rev. 65(1994)320-332.
10. GROENEN, G, JANSSENS, L., KAYEMBE, T, NOLEET, E., COUSSENS. L. and PATTYN, S. R. Prospective study on the relationship between intensive bactericidal therapy and leprosy reactions. Int. J. Lepr. 54(1986)236-244.
11. KATOCH, K., RAMANATHAN, U., NATRAJAN, M., BAGGA, A. K., BHATIA, A. S., SAXENA, R. K. and RAMU, G. Relapses in paucibacillary patients after treatment with three short-term regimens containing rifampin. Int. J. Lepr. 57(1989)458-464.
12. KIRKWOOD, B. R. Essentials of Medical Statistics. Oxford: Blackwell Scientific. 1988.
13. LIENHARDT, C. and FINE:, P. E. M. Type I reaction, neuritis and disability in leprosy. What is the current epidemiological situation? Lepr. Rev. 65(1994)190-203.
14. LOCKWOOD, D. N. J., VlNAYAKUMAR, S., STANLEY, J. N. A., MCADAM, P. W. J. and COLSTON, M. J. Clinical features and outcome of reversal (type I) reactions in Hyderabad, India. Int. J. Lepr. 61(1993)8-15.
15. NAAIS, B. Treatment of reactions and nerve damage. Int. J. Lepr. 64 Suppl. (1996)s21-s28.
16. NAAIS. B., MADOMBI, L., MATEMERA, B. O. and ELLIS, B. P. B. Short-term WHO advised multiple drug treatment of paucibacillary leprosy patients. Indian J. Lepr. 58(1986)348-353.
17. NAAIS, B., PKARSON, J. and Win vn. H. Reversal reaction: the prevention of permanent nerve damage. Comparison of short- and long-term steroid treatment. Int. J. Lepr. 47(1979)7-12.
18. PEARSON, J. The use of corticosteroids in leprosy. Lepr. Rev. 52(1981)293-298.
19. PONNIGHAUS, J. M. and BOERRIOTER, G. Are 18 doses of WHO/MDT sufficient lor multibacillary leprosy; results of a trial in Malawi. Int. J. Lepr. 63(1995)1-7.
20. PONNIGHAUS, I. M., BoLRRIGTER, G., FlNE, P. E. M., PONNIGHAUS, J. M. and RUSSELL, J. Disabilities in leprosy patients ascertained in a total population survey in Karonga District, northern Malawi. Lepr. Rev. 61(1990)366-374.
21. POST, E., CHIN-A-LIEN, R. A. M., BOUMAN, C, NAAIS, B. and FABER, W. R. Leprosy in The Netherlands in the period 1970-1991. Ned. Tijdschr. Geneeskd. 138(1994)1960-1963.
22. RICHARDUS, J. H., FLNLAY, K. M., CROFT, R. P. and SMITH, W. C. S. Nerve function impairment in leprosy at diagnosis and at completion of MDT: a retrospective cohort study of 786 patients in Bangladesh. Lepr. Rev. 67(1996)297-305.
23. RIDLEY, D. S. Reactions in leprosy. Lepr. Rev. 40(1969)77-81.
24. ROCHE, P. W., THEUVENET, W. J. and BRITTON, W. J. Risk factors for type-1 reactions in borderline leprosy patients. Lancet 338(1991)654-657.
25. ROSE, P. and WATERS, M. Reversal reactions in leprosy and their management. Lepr. Rev. 62(1992)113-121.
26. SCOLLARD, D. M., SMITH, T, BHOOPAT, L., THEEI KANONT, C, RANGDAENG, S. and MORENS, D. M. Epidemiologic characteristics of leprosy reactions. Int. J. Lepr. 62(1994)559-567.
27. SUGUMARAM, D. S. T. Steroid therapy for paralytic deformities in leprosy. Int. J. Lepr. 65(1997)337-344.
28. VAN BRAKEL, W. H. and KHAWAS, I. B. Silent neuropathy in leprosy: an epidemiological description. Lepr. Rev. 65(1994)350-360.
29. VAN BRAKEL, W. H. and KHAWAS, I. B. Nerve damage in leprosy: an epidemiological study of 396 patients in west Nepal-part 1. Definitions, methods and frequencies. Lepr. Rev. 65(1994)204-221.
30. VAN BRAKEL, W. H., KHAWAS, I. B. and LUCAS, S. Reactions in leprosy: an epidemiological study of 386 patients in west Nepal. Lepr. Rev. 65(1994)190-203.
31. VIJAYAKUMARAN, V., MANIMOZHI, N. and JESUDASAN, K. Incidence of late lepra reaction among multibacillary leprosy after MDT. Int. J. Lepr. 63(1995)18-22.
32. WATSON, J. M. Essential Action to Minimise Disability in Leprosy Patients. London: The Leprosy Mission, 1986.
33. WORLD HEALTH ORGANIZATION. A guide to leprosy control. 2nd cdn. Geneva: World Health Organization. 1988.
P. A. M. Schreuder, M.D., M.Sc., Leprosy Control Center Region 6, Khon Kaen, Thailand.
Reprint requests to Dr. Schreuder at his present address: NSL Medical Officer, East Java Leprosy Control Project, Provincial Health Office, Jl. Karangmenjangan No. 22, Surabaya 60285, Indonesia.
Received for publication on 3 February 1998.
Accepted for publication in revised form on 17 April 1998.