- Volume 66 , Number 2
- Page: 227–9
Leprosy and HIV infection in Bahia, Brazil
To the Editor:
Human immunodeficiency virus (HIV) infection progression is characterized by a gradual decrease of CD4+ T cells associated with the loss of host defenses against several pathogens and development of opportunistic infections. Since the protection against leprosy correlates with the expression of cell-mediated immunity, one could expect an increased number of leprosy cases among HIV-infected individuals. In Brazil, the number of registered cases of AIDS is continuously increasing. This is also a problem in Bahia, where leprosy is an endemic disease (prevalence rate per 10,000 was 3.91 in 1995). The aim of this study is to determine the possible association between new leprosy cases and HIV infection.
Newly diagnosed cases of leprosy who had attended the outpatient leprosy service of the Hospital Universitario Prof. Edgard Santos in Salvador, Bahia, Brazil, between March 1993 and May 1995 were enrolled in the present study. Additionally, sera obtained from leprosy patients and controls living at Irece, an endemic leprosy rural area in the center of Bahia, were also included. A case of leprosy was defined as an individual with newly diagnosed and previously untreated leprosy, aged between 1560 years, and a resident of Bahia. Up lo two controls living in the same area and/or healthy contacts were chosen for each patient. A standardized questionnaire was used to register gender, age, residence and high-risk behavior to HIV infection in the two groups, including sexual activity, intravenous drug use and blood transfusion.
Blood was obtained from consenting patients or controls and the serum was separated and kept at -20ºC until the realization of the assays to determine anti-HIV antibodies. All sera were tested with an enzyme linked immunosorbent assay (ELISA) for antibodies to HIV (HIV-l/HIV-2 ELISA test kit; Cambridge Biotech). The positive samples were further analyzed with a second ELISA (Enzygnost anti-HIV 1/2 plus; Behring), or with a solid-phase enzyme immunoassay (Immunocomb Bi-spot HIV1 and HIV2). The samples which gave negative results in the second ELISA and/or Immunocomb were considered negative for HIV infection. The samples that remained positive were tested by Immunofluorescence assay (IFA) or Western blot (WB) (Genelabs diagnostic HIV1 1.3, Cellular products) in order to confirm HIV seropositivity.
RESULTS AND DISCUSSION
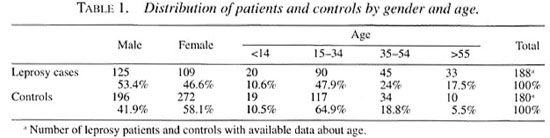
Sera from 234 patients and 468 controls were evaluated. Distribution by gender and age are shown in Table 1. Most of the patients (79.5%) and controls (867r>) lived in Salvador city and the remainder in rural areas.
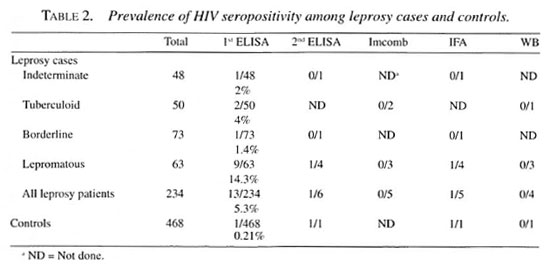
Case distribution according to leprosy classification and the results of HIV tests are shown in Table 2. Although 13 out of 234 patients and 1 out of 468 controls had at least one positive ELISA test, no positivity was confirmed by Immunocomb or WB. The majority (9/14) of our HIV-positive cases in the first ELISA test were from the Iepromatous leprosy group; only 1 was from the control group (Table 2).
HIV infection was not detected among newly diagnosed leprosy patients in Bahia in northeastern Brazil in this study. Other studies carried out in African countries and in some regions of India, where leprosy and AIDS are both endemic, did not show an association between the two diseases (1-3, 5-7). Another study conducted in Malawi found higher levels of HIV infection among newly diagnosed leprosy patients, but no clear association between HIV infection and leprosy incidence was found (8).
In some studies, HIV seropositivity was more frequent in multibacillary than in paucibacillary patients (1, 3, 6, 7); in other studies this was not found (2, 4, 5). We found a higher ratio of false-positive ELISA results for HIV antibodies in Iepromatous leprosy patients.
The reasons that may account for the unexpected lack of correlation between HIV infection and leprosy are not known. In our study, a majority of the patients and controls seemed to have very low behavioral risk for HIV infection as indicated by the questionnaire. In addition, most leprosy patients came from areas in which HIV prevalence is low. These factors may explain the HIV seronegativity found. Another way to examine this association should include determination of leprosy infection in newly diagnosed HIV-positive patients, but this is technically very difficult.
Our results suggest that in our region new leprosy cases are not associated with HIV infection. Larger studies performed in other areas endemic for leprosy in Brazil, where an increase of HIV infection has been detected, may help to better define the potential interaction between the two diseases in Brazil.
- Paulo Machado, M.D.
Yonara David, M.D.
Serviço de Imunologia
HUPES
Universidade Federal da Bahai
Rua João das Botas, s/n
40.110-040 SSA-Bahia, Brasil
-Celia Pedroso, B.Sc.
Carlos Brites, M.D.
HUPES
Universidade Federal da Bahai
SSA-Bahia, Brasil
-Aldina Barral, M.D.
Manoel Barral-Netto, M.D.
Serviço de Imunologia
HUPES
Universidade Federal da Bahai
SSA-Bahia, Brasil
and
Centro de Pesquisas Gonçalo Muniz
FIOCRUZ
SSA-Bahia, Brasil
Acknowledgment. We thank Dr. Howard Engers for his encouragement in performing this study. Mrs. Aldacy Andrade provided efficient support in the outpatient leprosy clinic. M. B.-N. and A. B. are Senior Investigators and Y. D. received a scientific initiation fellowship from CNPq (Brazilian National Research Council). This work was supported by a grant from the Special Programme for Research and Training in Tropical Diseases (TDR) of the UNDP/World Bank/World Health Organization.
REFERENCES
1. BORGDORFF, M. W., VAN DEN BROEK, J., CHUM, H. J., KLOKKE, A. H., GRAF, P., BARONOO, L. R. and NEWELL, J. N. HIV-1 infection as a risk factor for leprosy; a case-control study in Tanzania. Int. J. Lepr. 61(1993)556-561.
2. JAYASHEELA, M., SHARMA, R. N., SEKAR, B. and THYAGARAJAN, S. P. HIV infection amongst leprosy patients in south India. Indian J. Lepr. 66(1994)428-432.
3. KAWUMA, H. J., BWIRE, R. and ADATU-ENGWAU, F. Leprosy and infection with the human immunodeficiency virus in Uganda; a case-control study. Int. J. Lepr. 62(1994)521-526.
4. LIENHARDT, C, KAMATE, B., JAMET, P., TOUNKARA, A., FAYE, O. C, SOW, S. O. and BOBIN, P. Effect of HIV infection on leprosy: a three-year survey in Bamako, Mali. Int. J. Lepr. 64(1996)383-391.
5. MUNYAO, T. M., BWYAO, J. J., OWILI, D. W., NDINYA ACHOALA, J. O., KWASA, T. O. and KREISS, J. K. Human immunodeficiency virus-1 in leprosy patients attending Kenyatta National Hospital Nairobi. East Afr. Med. 71(1994)490-492.
6. OREGE, P. A., FINE, P. E. M., LUCAS, S. B., OBURA, M., OKELO, C. OKUKU, P. and WERE, M. A case-control study on human immunodeficiency virus-1 (HIV-I) infection as a risk factor for tuberculosis and leprosy in western Kenya. Tubere. Lung Dis. 74(1993)377-381.
7. SEKAR, B., JAYASHEELA, M., CHATTOPADHYA, D., ANANDAN, D., RATHINAVEL, L., VASANIHI, B., SUBRAMANIAN, M. and RAO, P. S. Prevalence of HIV infection and high-risk characteristics among leprosy patients of South India, a case-control study. Int. J. Lepr. 62(1994)527-531.
8. STERNE, J. A. C, TURNER, A. C, FINE, P. E. M., PARRY, J. V, LUCAS, S. B., PONNIGHAUS, J. M., MKANDWIRE, P. K., NYASULU, S. and WARNORFF, D. K. Testing for antibody to human immunodeficiency virus type I in a population in which mycobacterial diseases are endemic. J. Infect. Dis. 172(1995)543-546.
Reprint requests to Dr. Machado at the above address or FAX 55-71 -245-7110.