- Volume 66 , Number 2
- Page: 232–4
Giant lesions in histoid leprosy
To the Editor:
A 25-year-old woman from Bihar, India, an area endemic for leprosy, presented with papulonodular eruptions on the trunk and extremities of 3 years' duration. They had started as asymptomatic papules on the legs and slowly spread to the rest of the body. A cutaneous examination revealed well-demarcated, flesh-colored nodules, 5 cm in diameter bordered by a zone of hyperpigmen-tation on the lower aspect of both legs, more on the right side. A few nodules were subcutaneous in location. Five to six smaller discrete lesions were seen on the buttocks, trunk and the pinna of her right ear. Some of the bigger nodules on the legs showed erosion at the top, one of them being situated near a subcutaneous nodule (Fig. 1). The rest of the skin surface was unremarkable. Systemic examination revealed no abnormalities. Routine blood and urinalysis, liver and renal function tests, lipid profile, blood VDRL. ELISA for HIV and an X-ray of the chest were all negative or within normal limits. A biopsy from a truncal lesion revealed a thinned out epidermis with an underlying nodular infiltrate of spindle-shaped histiocytes arranged in whorls (Fig. 2). In some places the histiocytes showed a foamy cytoplasm. Dermal nerve twigs were seen amid the infiltrate of histiocytes and epithelioid cells. Using the Fite-Faraco stain, innumerable, rather elongated, acid-fast bacilli (AFB) were seen. They were occasionally arranged in clumps called globi.
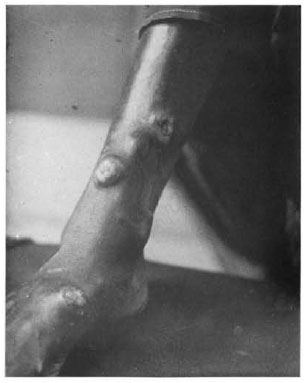
Fig. 1. Nodules on leg showing erosion.
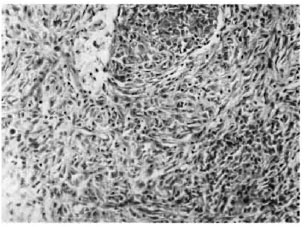
Fig. 2. Hypercellular lesion showing spindle-shaped cells in interlacing bundles and whorls (hematoxylin and eosin ×800).
Slit-skin smears from the nodules on the leg showed a bacterial index (BI) of 6+ (Ridley scale); those from the normal-appearing skin of the back and ears had a BI of 1+. The Mitsuda reaction to an intradermal lepromin test read after 4 weeks following the injection of 0.1 ml of armadillo-derived lepromin (Carville, Louisiana. U.S.A.) on the volar surface of the forearm was negative.
The patient was admitted to the hospital and given multidrug therapy recommended by the World Health Organization (WHO) (19) comprising once-monthly supervised rifampin 600 mg and clofazimine 300 mg orally, followed by dapsone 100 mg and clofazimine 50 mg every day. When visible improvement was seen as a fall in the BI and a reduction in the size of the nodules, she was discharged and treated as an outpatient. At the end of 2 years of therapy the BI had fallen to 2+, and she is continuing treatment.
DISCUSSION
The term histoid leproma, in contrast to the classical leproma of leprosy, was put forward as a histologic concept by Wade in I960 (l7) to describe a bacillus-rich lesion composed of spindle-shaped cells resembling fibrocytes in which globi were not seen. In a detailed report (18) the lesions, either limited or many and affecting the face, trunk and extremities, were found to be mainly subcutaneous in origin giving rise to nodules; the other lesions arising from the dermis were protuberant or pedunculated nodules and thick scaly plaques at the pressure points. The nodules may at times arise from the peripheral nerves (10). Lesions of histoid leprosy are soft-to-firm, reddish, dome-shaped and shiny due to the stretching of the skin by the expanding granuloma. Later the skin may give way, resulting in shallow ulcers. Early lesions are soft and succulent but fibrosis increases with the age of the lesion making it linn. Umbilicated papules mimicking molluscum contagio-sum may be seen (11). The size of the lesion varies from 0.5 cm to 4 cm (1). Occasionally, pad-like plaques can give rise to big nodules in the loose skin around the major joints (6). Histoid leprosy was initially seen in patients with a previous history of lepro-matous leprosy (LL), and they were unresponsive to dapsone (l8). The important observation of dapsone resistance was later proved experimentally (7). Subsequent reports have shown that histoid leprosy can arise in long-standing LL or borderline lep-romatous leprosy with or without previous dapsone therapy (3, 9, 15), or de novo (11). Although histoid leprosy is considered to be a form fruste of LL, the salient clinical features that distinguish it from classical LL are that the nodules, being expansile and not infiltrative, arise from the apparently normal skin and there is no madaroris. In one series on histoid leprosy with significant facial involvement, the ears and nasal mucosa showed little change, the larynx was spared, and none showed destruction of the nasal cartilage even after years of uncontrolled disease (9). However, the relative absence of the occurrence of type 2 lepra reactions and the absence of globus formation are not always seen.
The notable histopathological findings are the presence of spindle-shaped histiocytes in the dermal infiltrate arranged in intertwining and whorled patterns. They contain numerous bacilli and the cytoplasm may be slightly vacuolated but lacks the marked vacuolation seen in lepra cells (5). The bacilli are intact and rod-shaped in contrast to the fragmented forms seen in conventional LL (5), a feature described as the histoid habitus (l8). Other large series show that classical histopathological features are seen in approximately one quarter of the patients with the clinical picture of histoid leprosy (4) and vary from well-circumscribed. tumorous, nonvacuolated, spindly histiocytic collections separated by collagen fibers to a circumscribed mass of foamy cells akin to that seen in LL (1). Other features, such as epidermal atrophy and a free subepidermal zone, are seen in both histoid leprosy and LL. The significance of the histoid transformation in LL remains unclear. It may represent a proliferation of sulfone-resistant mutants of Mycobacterium leprae after the elimination of the susceptible ones by therapy (15). Since a good number of patients respond to dapsone, the hyperactive cellular response seen in histoid leprosy may be a tissue reaction to continuous stimulation by M. leprae in long-standing LL similar to that seen with chronic inflammation (4). or it may denote an immune response to a second infection in a person who has acquired some resistance from the previous episode of LL (14). The occasional presence of AFB-negative tuberculoid foci in histoid lesions (8) may be a heightened tissue response to the large number of organisms (7), and suggests abortive attempts to clear the infection. An immunological study has also shown a relatively enhanced cell-mediated immunity in histoid as compared to active nonhistoid LL (16).
In our patient, the bigger nodules showing reactive hyperpigmentation tit the base were present on the legs. This also suggests a longer duration of the lesions which the patient may either not have noticed or have given misinformation. The other lesions were scattered over the trunk and buttocks, and the lace was less affected. The Bl from the lesions was 6+. The apparently normal skin which usually shows no organisms had a low BI of 1+; this can happen sometimes in patients with histoid leprosy (l5). She responded satisfactorily to the multidrug regimen for leprosy. Since M. leprae can only be grown in the mouse foot pad and not cultured, if resistance is suspected it is usually confirmed clinically by giving regular and supervised dapsone in full dosage for 6 months; if the morphological index (a count of only the solid-staining bacilli) does not fall to near 0, the organisms are considered to be insensitive (2). Being uncommon, histoid leprosy can easily be mistaken for neurofibroma, dermatofibroma and/or related tumors. The clustering of lesions in the center of the face bears a striking resemblance to post-kala-a/.ar dermal leishmaniasis (12). A slit-skin smear is a simple office procedure to eliminate these conditions.
- V. Ramesh, M. D.
R. S. Misra
Dilip Kachhawa
Sudhir B. Kulkami
Department of Dermatology and Institute of Pathology (ICMR)
Safdarjang Hospital
New Delhi, India
REFERENCES
1. BHUTANI, L. K., BEDI, T. R., MALHOTRA, Y. K., KANDIIARI, K. C. and DEO, M. G. Histoid leprosy in North India. Int. j. Lepr. 42(1974)174-181.
2. BRYCESON, A. D. M. and PFALTZGRAFF, R. E. Leprosy . Edinburgh: Churchill Livingstone, 1990, pp. 80-81.
3. CHAUDHURY, D. S., CHAUDHURY, M. and ARMAH, K. Histoid variety of lepromatous leprosy. Lepr. Rev. 42(1971)203-207.
4. DESIKAN, K. V. and IYER, C. G. S. Histoid variety of lepromatous leprosy; a histopathologic study. Int. j. Lepr. 40(1972)149-156.
5. MANSEIELD, R. C. Histoid leprosy. Arch. Pathol. 87(1969)580-585.
6. PAVITHRAN, K. Giant histoid tumor. Int. j. Lepr. 60(1992)274-276.
7. PETTIT, J. H. S., REES. R. J. and RIDLEY, D. S. Studies on sulfone resistance in leprosy. I. Detection of cases. Int. j. Lepr. 34(1966)375-390.
8. PORICHHA, D. and BHATIA, V. N. Epithelioid and polygonal cells in histoid leprosy. Indian j. Lepr. 59(1987)191-193.
9. PRICE:, E. W. and FITZHERBERT, M. Histoid (high-resistance) lepromatous leprosy. Int. j. Lepr. 34(1966)367-374.
10. RAMANUJAM, K., ARUNTHATHI, S., CHACKO, C. J. G. and JACOB, M. 'Neural histoid.' Histoid leproma in a peripheral nerve; a case report. Lepr. Rev. 55(1984)63-68.
11. RAMANUJAM, K. and RAMD, G. Wade's histoid lepromatous leprosy; report of a clinical study. Lepr. India 41(1969)293-297.
12. RAMESH, V. On the difference between post-kala-a/.ar dermal leishmaniasis and leprosy. Trop. Doe. 24(1994)120-121.
13. RAMESH, V. and ZAHLER, S. A. Umbilicated lesions in leprosy. Int. j. Dermatol. 34(1995)295-296.
14. RIDLEY, D. S. Histological interpretation and clinical application. In: Skin Biopsy in Leprosy . Basle: Ciba-Geigy, 1977, pp. 34-35.
15. RODRIGUEZ, J. N. The histoid leproma; its characteristics and significance. Int. j. Lepr. 37(1969)1-21.
16. SEIK.AL, V. N., Srivastava, G. and Sana, K. Immunological status of histoid leprosy. Lepr. Rev. 56(1985)27-33.
17. WADE, H. W. The histoid leproma. Abstract. Int. j. Lepr. 28(1960)469-470.
18. WADE, H. W. The histoid variety of lepromatous leprosy Int. j. Lepr. 31(1963)129-142.
19. WHO STUDY GROUP. Chemotherapy of leprosy for control programmes. Geneva: World Health Organization, 1982. Tech. Rep. Ser. 675.
Reprint requests to V. Ramesh, M. D., Sector 12/1082, RK Puram, New Delhi 110 022, India.