- Volume 66 , Number 2
- Page: 235–8
Serologic assays using the 18-kDa antigen of Mycobacterium leprae expressed in the yeast Saccharomyces cerevisiae
To the Editor:
Leprosy diagnosis is mainly based on clinical data and is confirmed by the detection of Mycobacterium leprae in infected tissues by direct bacilloscopy. In tuberculoid (TT) cases, the bacilli are seldom found and the diagnosis can only be confirmed by histopathologic examination. The development of specific serological assays for easier etiological diagnosis would allow the realization of seroepidemiological assays and would improve the follow up of patients, which possibly would imply in the installation of earlier and more effective control measures (8).
Among different M. leprae antigens, special attention was given to the 18-kDa antigen (p18), since it was involved in the immune response in mice and in humans (4, 7) and was initially considered restricted to two mycobacteria species, because cross-reaction using a specific monoclonal antibody was only found with M. simiae serovar 1 (12). Our group have developed a system for the expression of p18 in Saccharomyces cerevisiae utilizing an inducible promoter and a secretion cassette that produces more than 100 ing/L of pi 8 (18). This antigen was purified and used for delayed-type hypersensitivity assays in mice (19). In the present report, the use of this purified recombinant antigen in enzyme immunoassays (ELISA) was verified for the detection of M. leprae infections.
We studied seven leprosy outpatients followed up at the Department of Dermatology from São Paulo State Health Secretary at São Paulo, Brazil. The bacterial index (BI) (1-1-5+) was determined by analysis of the earlobe exudate and the clinical form was classified according to the criteria proposed by Ridley and Jopling (20). For our control group, we used eight confined individuals from a psychiatric hospital at Juqueri (about 30 km from São Paulo) for whom no previous contact with leprosy patients had been reported. The Brazilian Ministry of Health ethics guidelines were followed.
RESULTS AND DISCUSSION
Converse to previous serological assays, in this study we analyzed the use of an antigen corresponding to the authentic p18 expressed in S. cerevisiae for the detection of specific antibodies. This study is also the first to analyze the detection of anti-p18 antibodies among Brazilian patients.
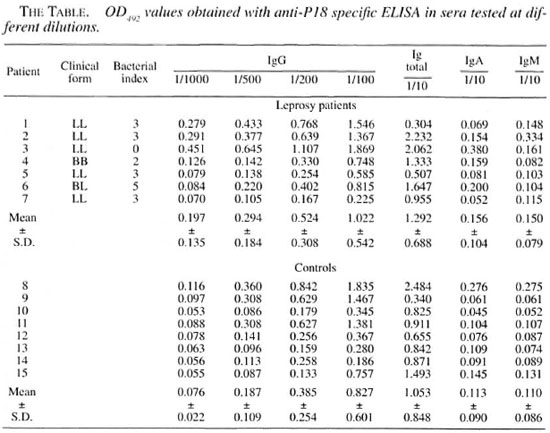
The results of the ELISAs for the detection of anti-p18 antibodies (total and classes IgG, IgM and IgA) in the leprosy patients and the control group are shown in The Table. For total antibodies, IgA and IgM classes, patient sera were diluted 1/10.
For the IgG class, serum dilution ranged from 1/100 to 1/1000. The means of the OD492 values obtained from leprosy patients were always higher than those obtained from the control group, independent of the antibody class analyzed, but no statistically significant differences were found (Student's t test). The largest difference between the mean value in patients and in the control group was obtained when the patient sera was diluted 1/1000. Some individuals in the control group also showed higher OD492 values.
Other studies have shown that p18 would not be suitable for a routine serological assay for the diagnosis or follow up of leprosy patients since many patients (especially at the tuberculoid pole) did not show anti-p18 antibodies but some control patients also showed these antibodies (9, 11, 21, 22).
These previous results led us to first check only patients closer to the lepromatous pole of the disease. We observed that the average levels of antibodies against p18 in these patients were generally higher than those of the control group. However, some leprosy patients did not show anti-p18 anti-bodies and some individuals in the control group did have high antibodies levels against p18.
Initial reports describe this antigen restricted to a few mycobacterial species, such as M. siniiae serovar 1 (12) and M. smegmatis (15). Later, it was found that crossreaction could also be found with M. tuberculosis and M. bovis BCG (5, 6, 21, 22). These data were not confirmed by other authors, especially at the T-cell level (16, 17, 18), and no homologous gene was found in M. tuberculosis even when using polymerase chain reaction (PCR) with degenerated primers (3). Interestingly, the presence of T-cell clones recognizing specifically p18 from M. leprae (but not from other mycobacteria species) was recently reported from one subject who was not exposed to leprosy (1). The nature of the antigen stimulating this apparent specific response could not be determined.
On the other hand, the absence of detectable anti-p18 antibodies in some leprosy patients can be explained by the fact that the response against this protein in the natural infection seems to be more important at the cellular level (17) and is not an early event (13).
It is noteworthy that the patient who showed the highest antibody levels for all of the immunoglobulins tested (except IgM, for which he showed the second highest antibody level tested) was also the only one analyzed who showed a negative result on the bacilloscopy assay, although he was classified as LL. This result agrees with some data previously published suggesting that p18 would elicit a protective response against M. leprae infection (6). It was recently shown that immunization with recombinant BCG expressing p18 was capable of inducing antibodies, a lymphocyte proliferative response, and some protection against infection (2).
In conclusion, the 18-kDa recombinant antigen of M. leprae obtained in S. cerevisiae does not seem useful for routine serological assays for the diagnosis or follow up of leprosy patients.
- João R. R. Pinho, M.D., Ph.D.
Vanda A. U. F. de Souza, Ph.D.
Heitor F. Andrade, Jr., M.D., Ph.D.
Ana Clara G. Schenberg, Ph.D.
Laboratório de Biologia Molecular Instituto Adolfo Lutz
Laboratórios de Virologia e de Protozoologia, Instituto de Medicina Tropical de Sao Paulo, FMUSP and Centro de Pesquisas em Biotecnologia-ICBUSP
Sao Paulo, SP, Brasil
Acknowledgment. This work was supported by the World Health Organization/Tropical Diseases Research Program/World Hank, the Centro Brasileiro Argentino de Biotecnologia, LIMHCFMUSP and CAPES.
REFERENCES
1. ADAMS, E., BASTEN, A., PRESTIDGE. R. and BRITTON, W. J. T cell clones front a non-leprosy exposed subject recognize the Mycobacterium leprae lS-kD protein. Clin. Exp. Immunol. 102(1995)58-64.
2. BAUMGART, K. W., MCKENZIE, K. R., RADIFORD, A. J., RAMSMAW. I. and BRITTON, W. J. Immunogenicity and protection studies with recombinant mycobacteria and vaccinia vectors coexpressing the 18-kilodalton protein of Mycobacterium leprae. Infect. Immun. 64(1996)2274-2281.
3. BOOTH. R. J., WILLIAMS, D. L., MOUDGIL, K. D., NOONAN, L. C, GRANDISON, P. M, MCKEE, J. J., PR ESTIDGE, R. L. and WATSON, J. D. Homologs of Mycobacterium leprae 18-kilodalton and Mycobacterium tuberculosis 19-kilodalton antigens in other mycobacteria. Infect. Immun. 61(1993)1509-1515.
4. BRITTON, W. J., HELLQVIST, L.,. BASTEN, A. and RAISON, R. L. Mycobacterium leprae antigens involved in human immune responses: I. Identification of four antigens by monoclonal antibodies. J. Immunol. 135(1985)4171 -4177.
5. DOCKRELL, H. M. STOKER, N. S., LEE, S. P., JACKSON, M., GRANT. K. A., JOUY. N. P., LIXAS, S. B., HASAN, R., HUSSEIN, R. and MCADAM. K. P. T-cell recognition of the 18 kilodalton antigen of Mycobacterium leprae. Infect. Immun. 57(1989)1535-1541.
6. DOHERTY.T. M., BOOTH. R. J., LOVE, S. G., GIBSON, J. J., HARDING, R. K. and WATSON, J. D. Characterization of an antibody-binding epitope from the 18kl)a protein on Mycobacterium leprae. J. Immunol. 142(1989)1691-1695.
7. ENGERS, H. D., ABE, M., BLOOM, B. R., MEHRA, V., BUCHANAN, T. M., KHANOLKAR, S. K., YOUNG, D. B., CLOOS. O., GILLIS, T. HARBOE, M., IVANYI, J., KOLK, A. H. J. and SHEPARD, C. C. Results of a World Health Organization sponsored workshop on monoclonal antibodies to M. leprae. Infect. Immun. 48(1985)603-605.
8. HASTINGS, R. C. GILLIS. T. P., KRAHENBUHL, J. L. and FRANZBLAU, S. G. Leprosy. Clin. Microbiol. Rev. 1(1988)330-348.
9. HUSSAIN, R., DOCKRELL, H. M., KIFAYET, A., DAUD. A., WATSON, J. D., CHIANG, T. J. and STOKER, N. G. Recognition of Mycobacterium leprae 18-kDa protein in leprosy. Int. J. Lepr. 60(1992)368-375.
10. ILANGUMARAN, S., SHANKERNAYAN, N., RAMU, G. and MUTHAKKARPPAN, V. Antibody response to recombinant 65-kDa, 70-kDa and 18-kDa mycobacterial antigens in leprosy patients and healthy contacts in a leprosy endemic population. Int. J. Lepr. 62(1994)245-255.
11. KUAN, M. B., DESHPANDE, R. G, DAVIDSON, S. K. and NAVALKAR, R. G. Sero-imniunoreaetivity of cloned protein antigens of Mycobacterium leprae. Int. J. Lepr. 60(1992)195-200.
12. LAMB, F. I., SINGH. F. I. and COLSTON, M. J. The specific 18-kilodalton antigen of Mycobacterium leprae crossreacts with Mycobacterium habana and functions as a heat-shock protein. J. Immunol. 144(1990)1922-1925.
13. LAUNOIS, P., NIANG N'DIAYE, M., SARTHOU, J. L., DROWART, A., VAN VOOREN. J. P., CARTEL, J. L. and HUYGEN, K. T cell reactivity against antigen 85 but not against the 18- and 65-kD heat shock proteins in the early stages of acquired immunity against M. leprae. Clin. Exp. Immunol. 96(1994)86-90.
14. MUSTAFA. A. S. Identification off-cell activating recombinant antigens shared among three candidate antileprosy vaccines, killed M. leprae. M. bovis BCG and Mycobacterium w. Int. J. Lepr. 56(1988)265-273.
15. MUSTAFA, A. S., GILL. H. K.,. NERI and. A., BRITTON, W. J., BEHRA, V., BLOOM. B. R., YOUNG, R. A. and GODAL, T. Human T-cell clones recognize a major M. leprae protein antigen expressed in E. coli. Nature 319(1986)63-66.
16. MUSTAFA, A. S., LUNDIN, K. E. A. and OFTUNG, F. Human T cells recognize mycobacterial heat shock proteins in the context of multiple HLA-DR molecules: studies with healthy subjects vaccinated with Mycobacterium bovis BCG and Mycobacterium leprae. Infect. Immun. 61(1993)5294-5301.
17. MUSTAFA, A. S. and OFTUNG, F. Long lasting T cell reactivity to Mycobacterium leprae antigens in human volunteers vaccinated with killed M. leprae. Vaccine 11(1993)I 108-1 IIS.
18. PINHO, J. R. R., BARR, P. J., VICENTE, E. J. and SCHENBERG, A. C. Expression of the 18kDa protein of Mycobacterium leprae in Saccharomyces cerevisiae . Biotech. Lett. 16(1994)1241-1 24b.
19. PINHO, J. R. R., CARDI, B. A., ANDRADE JR., H. F., BARR, P. J., BARTHURST, I. C., VICENTE, E. J. and SCHENBERG, A. C. Immunogenic properties of the Mycobacterium leprae recombinant 18kDa antigen purified from Saccharomyces cerevisiae -enhancement of delayed type hypersensitivity after gamma-irradiation. Int. J. Lepr. 63(1995)381-390.
20. RIDLEY, D. S. and JOPLING, W. H. Classification ofleprosy according to immunity; a live-group system. Int. J. Lepr. 34(1966)255-273.
21. ROUCHE, P. W., PRESTIDGE, R. L. WATSON, J. D. and W. J. Antibody responses to the 18-kDa protein of Mycobacterium leprae in leprosy and tuberculosis patients. Int. J. Leff. 60(1992)201-210.
22. VIKEFORS, T., OLCÉN, P., WIKER, H. and WATSON, J. D. Serological response in leprosy and tubercu-losis patients to the 18-kDa antigen of Mycobacterium leprae and antigen 85B of Mycobacterium bovis BCG. Int. J. I.epr. 61(1993)571-580.
23. WORLD ORGANIZATION. Progress towardselintinating leprosy as a public health problem.Wkly. Epidemiol. Rec. 69(1994)145-152.
Reprint requests to Dr. J. R. R. Pinho, Laboratório de Biologia Molecular, Instituto Aldolfo Lutz, Avenida Dr. Arnaldo 355, 012246-902 Sao Paulo, SP, Brasil, email: jrrpinho@usp.br; fax: 55-11-853-3505.