- Volume 66 , Number 2
- Page: 238–40
Collagen profile in sciatic nerves of M. leprae-inoculated mice correlates with in vitro collagen production by schwann cells
To the Editor:
Peripheral nerves are tissues especially rich in their collagen content, with the prominent collagen types being I, III, IV and V (8). Histopathological observations of nerves from leprosy patients reveal increased collagen deposition in the early stages and the inflammatory cell populated later stages (6-7). Sciatic nerves from experimentally infected Swiss white (SW) mice also show the presence of collagen pockets, indicative of freshly laid down matrix, around unmyelinated fibers (9, 10). This has been corroborated by in vitro studies where Schwann cells, the major producer of collagen in the peripheral nerve (5), from SW mice have been shown to produce increased levels of different collagen types on infection with Mycobacterium leprae (11). The SW mouse is a strain in which the response of host cells to M. leprae infection parallels those observed in lepromatous patients (3, 4) as opposed to the C57BL/6 mouse, a strain in which the response to M. leprae parallels that observed in tuberculoid patients or normal individuals (3, 4). In vitro , Schwann cells from C57BL/6 mice exhibit unaltered collagen metabolism on infection with M. leprae (11). Expression of collagen types I, III, and IV, the most abundant collagen types found in peripheral nerves, was therefore studied using indirect immunoperoxi-dase staining to determine its correlation with in vitro observation on collagen production by neural cell population.
The SW and C57BL/6 strains of mice were inoculated with 104 M. leprae /foot pad. Sciatic nerves were collected from the animals at months 12 and 20 postinfection. At each time interval, sciatic nerves from age-matched, uninfected control mice were also collected. The nerves were placed in Formal-Zenker fixative and processed for paraffin blocks. Slides containing transverse and longitudinal nerve sections (5-um thick) were dewaxed by treating with xylene. The sections were rehydrated by passing through graded ethanol and treated with Lugol's iodine and sodium thiosulfate. The sections were then treated with 3% hydrogen peroxide for 15 min for nonspecific peroxidase and preblocked with 1% fetal calf serum for 30 min at 37ºC. The sections were then treated with a 1:5() dilution of antibodies raised in goat against human collagen types I and III (Sera-lab, code 1310 and 1330, respectively) for 3 hr at 37ºC. Primary antibody minus sections for each nerve were included as negative controls. Following washes with TBS (Tris buffered saline containing 0.05% Tween), the sections were incubated with a 1:2() dilution of horseradish peroxidase conjugated anti-goat immunoglobulin for 20 mill at 37ºC. Following washes with TBS, the sections were treated with substrate (6 mg di-aminobenzidine + 30 (il of 3% hydrogen peroxide in 10 ml of TBS) and counter-stained with Harris hematoxylin. The intensity of immunostaining for different collagen types was assessed visually and expressed subjectively as absent (-), low (1+), moderate (2+), high (3+) and extremely high (4+). The observations and grading were made in relation to each of the following regions: a) endoneurium, b) epineurium, c) perineurium and d) around Schwann cells/axons. A collagen profile around the blood vessels could not be ascertained since they could not be spotted uniformly in all of the nerves. The immunoperoxidase staining intensity of the three collagen types in the different neural regions of mouse nerve has been summarized in The Table.
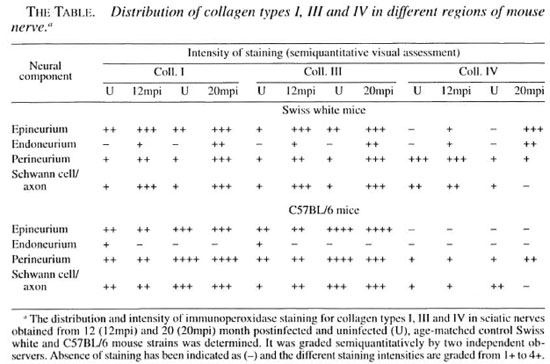
The epineurium in nerves of age-matched, uninfected SW and C57BL/6 mouse nerves was positive for collagen types I and III at both month 12 and month 20, while no positivity for collagen type IV was observed in the epineurium. The endoneurium, on the other hand, was either negative or showed low positivity for the three collagen types in both SW and C57BL/6 control nerves at both 12 and 20 months. The perineurium was positive for collagen types I, III and IV at both time intervals in SW and C57BL/6 control nerves. Similarly, positivity for collagen types I, 111 and IV was also observed around Schwann cells in both SW and C57BL/6 strains. In addition, the general intensity for staining in all of the areas was higher in C57BL/6 mice compared to the SW strain.
In nerves from infected C57BL/6 sciatic nerves, the staining intensity of collagen types I, III and IV in the different neural regions was generally comparable to the nerves of age-matched control animals at all postinfection time periods. However, in nerves from infected SW mice, an increase in staining intensity of collagen types I and III was observed in the epineurium, perineurium and around Schwann cells. The three collagen types were also observed at a higher intensity in the endoneural spaces at month 12 postinfection compared to the nerves from age-matched control animals. In contrast, staining for collagen type IV in the perineurium and around Schwann cells, which was observed in nerves from controls and 12-month postinfected mice, declined and was low by month 20 postinfection.
The results indicate a good correlation in the collagen profiles of sciatic nerves from M. leprae -'mfecied SW and C57BL/6 mice and the previous in vitro observations on collagen production by M. leprae -infected Schwann cells from the two strains of mice. Similar to the in vitro studies, where Schwann cells from SW mice increased synthesis of collagen types I, III, and IV in response to M. leprae infection (11), nerves from infected SW mice showed an increase in staining intensity for the different collagens, indicating increased deposition of the same. The observation of unchanged im-munoperoxidase staining intensities in controls and infected C57BL/6 nerves also was similar to the in vitro observations of unchanged collagen production by M. leprae -infected Schwann cells from the same strain (11). This indicates that collagen metabolism is not excessively disturbed in this strain.
Metabolic alteration in Schwann cells as a consequence of M. leprae infection has been implicated as a major factor in the initiation and development of leprous nerve pathology (1, 2). The present study, in combination with the previous in vivo and in vitro observations (9, 11), adds strength to the view that Schwann cells represent the initial site of the lesion from which the nerve pathology progresses to the later stages of nerve damage. The present observations are of special significance since the nerve changes observed in foot pad-inoculated mice occur in the absence of inflammatory cells, a situation similar to the very early stages of nerve damage in leprosy patients in whom Schwann cell involvement is the prime feature.
- Neeta Singh, M.Sc.
Research Student
- Tannaz J. Birdi, Ph.D.
Senior Research Officer
- Noshir H. Antia, F.R.C.S.,
F.A.C.S. (Hon.)
Director and Trustee
The Foundation for Medical Research
84-A, R.G. Thadani Marg
Worli, Bombay 400 018, India
Acknowledgment. We thank llarish Pujari lor the technical assistance provided by him for the study. This work was funded by a grant (No. 030074/Z/89/Z) from The Wellcome Trust, London. Neeta Singh was supported by a fellowship (9/626(1)/91/EMR-l) from the Council for Scientific and Industrial Research. Government of India.
REFERENCES
1. ANTIA, N. H. Leprosy: a disease of the Schwann cell. Lepr. India 54(1982)599-604.
2. ANTIA, N. H, SHETTY, V P and METHA L. N. Study of the evolution of nerve damage in leprosy: part IV. An assessment. Lepr. India 52(1980)4S-52.
3. BlRDI, T. J. and ANTIA. N. H. The macrophage in leprosy: a review on the current status. Int. J. Lepr. 57(1989)511-525.
4. BIRDI, T. J., SALGAME, P. R. and ANTIA, N. H. The role of macrophages in leprosy as studied by protein synthesis of macrophages from resistant and susceptible hosts-a mouse and human study. Lepr. India 51(1979)23-42.
5. BUNGE, M. B., WILLIAMS, A. K., WOOD, P. M., USINO, J. and JEFFERY J. J. Comparison of nerve cell and nerve cell plus Schwann cell cultures, with particular emphasis on basal lamina formation. J. Cell. Biol. 84(1980)184-202.
6. JOB, C. K. Pathology of peripheral nerve lesions in lepromatous leprosy: a light and electron microscopy study. Int. J. Lepr. 39(1971)251-268.
7. JOB, C. K. Mechanisms of nerve destruction in tuberculoid borderline leprosy-an electron microscopy study. J. Neurol. Sei. 20(1973)25-32.
8. SHELLSWELL, G. B., RESTALL D. J., DUANCE, V. C. and BAILEY, A. J. Identification of differential distribution of collagen types in the central and peripheral nervous system. FEBS Lett. 106(1979)305-308.
9. SHETTY, V. P. Pathology of nerve damage ill leprosy. In: The Peripheral Nerve in Leprosy anil Associated Peripheral Neuropathies . Antia. N. H. and Shetty. V. P., eds. 1st edn. Delhi: Oxford University Press, 1997, pp. 79-137.
10. SINGH V. V. P., ANTIA, N. H. and JACOBS. J. M. The pathology of early leprous neuropathy. J. Neurol. Sci. 88(1988)115-131.
11. SINGH, N., BIRDI, T. J., CHANDRASHEKAR, S. and ANTIA, N. H. Schwann cell extracellular matrix protein production is modulated by M. leprae and macrophage secretory products. J. Neurol. Sci. 151(1997)13-22.
Reprint requests to Dr. Birdi at the above address; fax 91-22-266-2735; tel: 91-22-4934989/493-2876.