- Volume 66 , Number 3
- Page: 316–27
Delayed-type hypersensitivity reactions followed by erythema nodosum leprosum
ABSTRACT
Reported herein are 13 borderline lepromatous (BL) or subpolar lepromatous (LLs) patients who presented with or developed delayed-type hypersensitivity (DTH) reactions after initiation of antibacterial therapy, but who subsequently developed erythema nodosum leprosum (ENL), the DTH to ENL group. During the same time, three LLs patients had ENL followed by relapse-associated DTH, a significant (p <0.05) difference in sequence of the two conditions. The DTH to ENL group had statistically significant higher biopsy indexes at the time of diagnosis of the DTH reaction compared with two DTH control groups, 7 multibacillary patients presenting with DTH reactions and 15 BL or LLs who developed DTH reactions after starting treatment but had no ENL. DTH-associated histologic changes were less well developed in the DTH to ENL group than in either of the two control groups. In the DTH to ENL group, 77% required prednisone in addition to thalidomide to achieve a complete remission in contrast to only 10% of 21 ENL clinical controls. In the DTH to ENL group, the classical histologic ENL pattern was present in only 31 % of these patients, in contrast to 88% of 33 ENL histologic controls. In 9 of 9 of the DTH to ENL patients studied, after the ENL remitted, Mycobacterium leprae-sonicate-stimulated lymphocyte transformation tests gave stimulation indexes within the range of our tuberculoid (TT) and borderline tuberculoid (BT) patients, in contrast to absent responses in 6 ordinary, longterm-treated patients who had had ENL.RÉSUMÉ
Nous rapportons ici 13 patients lépromateux borderlines (BL) ou lépromateux (LLs) qui présentaient au premier examen clinique ou ont développé une hypersensitibilité retardée (HSR) après lŽadministration du traitement antibactérien, et qui one ensuite développé un érythème noueux lépreux (ENL), le groupe HSR-ENL. Durant le même temps, trois patients LLs ont eu un ENL suivi d'une HSR associée à une rechute, une différence significative (p <0.05) dans la séquence de ces deux conditions. Le groupe HSR-ENL avait un index de biopsie statistiquement plus élevé au moment du diagnostic de HSR que 2 groupes contrôles HSR. représentés par 7 patients miultibacillaires qui présentaient des réactions de type HSR et 15 BL ou LLs qui ont développé une HSR après le début du traitement mais n'ont pas souffert d'ENL. Les lésions histologiques associées à l'HSR du groupe HSR-ENL étaient moins importantes que celles observées dans les 2 groupes contrôles. Parmi le groupe HSR-ENL. 77% ont dû être traités par de la prednisone en plus de la thalidomide pour obtenir une rémission complète. Cela contraste avec seulement 10% parmi 21 contrôles souffrant cliniquement d'ENL. L'aspect histologique classique de l'ENL n'était d'aspect classique parmi 33 contrôles histologiques ENL. Des tests de transformation lymphoblastiques à partir de stimulations par des sonicats de Mycobacterium leprae ont été effectués chez neuf cas d'HSR-ENL, après rémission de l'ENL. Les indexs de stimulation obtenus à partir de cas 9 cas étaient de l'ordre de grandeur de ceux obtenus chez les patients tuberculoides (TT ) et borderline truberculoïdes (BT), se démarquant des réponses négligeables observées chez 6 patients traités au long cours qui avaient développé un ENL.RESUMEN
Se reportan 13 pacientes con lepra pre-lepromatosa (borderline, BL) o lepromatosa subpolar (LLs) que desarrollaron reacciones de hipersensibilidad tip tardio (DTH) después de iniciar el tratamiento antileproso y que luego desarrollaron eritema nodoso leproso (ENL). Este grupo se describe como el grupo DTH a ENL. En el mismo lapso del estudio, tres pacientes LLs presentaron ENL y luego desarrollaron DTH asociada a una recaída. El grupo DTH a ENL tuvo índices de biopsia significativamente más altos al tiempo del diagnóstico de la reacción DTH que los índices de biopsia encontrados en 2 grupos DTH control: 7 pacientes multibacilares que presentaron reacciones DTH y 15 pacientes BL o LLs quienes desarrollaron reacciones DTH después de iniciar el tratamiento pero que no tuvieron ENL. Los cambios histológicos asociados a DTH estuvieron menos desarrollados en el grupo DTH a ENL que en cualquiera de los 2 grupos control. En el grupo DTH an ENL el 77 % de los pacientes requirieron prednisona además de talidomida para alcanzar la remisión completa, en contrast con sólo el 10 % de 21 controles con ENL clínico. En el grupo DTH a ENL el patrón histológico clásico del ENL estuvo presente solo en el 31 % de los pacientes, en contraste con el 88 % de 33 controles con ENL histológico. En 9 de los 9 pacientes DTH a ENL estudiados, se encontró, después de que el ENL hubo remitido, que la estimulación de lifoeitos con un sonicado de Mycobacterium leprae dio índices de estimulación dentro del rango encontrado en los pacientes tuberculoides (TT) y pre-tuberculoides (BT), en contraste con la ausencia de respuesta en 6 pacientes con lepra tratada por mucho tiempo que sólo habían tenido ENL.The reactional states of leprosy command attention for a number of reasons. For the patients reactions are important causes of morbidity, for the clinicians reactions are therapeutic challenges, and for the immunologists reactions are probes for a better understanding of adoptive immunity in people. Jopling's type l reaction, also called a delayed-type hypersensitivity (DTH) reaction, if named by its putative mechanism, or called a reversal (upgrading) reaction or a downgrading reaction, if named by its observed outcome, occurs throughout the granulomatous spectrum of leprosy except at its polar extremes (because it is outcome-neutral, "DTH" will be used in this paper). In contrast, Jopling's type 2 reaction, descriptively named erythema nodosum leprosum (ENL), is restricted to multibacillary patients, borderline lepromatous (BL) or lepromatous (LL), whether polar (LLp) or subpolar (LLs) in type.
We have observed and report here on 13 patients who presented with, or developed on treatment, DTH reactions, but who subsequently developed ENL. Patients with both DTH and ENL reactions have been reported previously. For example, Scollard, et al. (17) mentioned two patients with both (occurring sequentially, personal communication). Also, among the eight LL patients who relapsed as BT, reported by Waters and Ridley (20) and by Girdhar, et al. (5), were two patients with ENL occurring in their lepromatous stage and DTH in their relapsing BT phase. In at least six patients, DTH and ENL reactions have been reported as occurring "simultaneously" (6) or in "a combined RR and ENL reaction" (2). We offer the hypothesis that the DTH to ENL patients reported here are a small but characteristic subset of patients who describe an extreme variety and liability of host responses toward Mycobacterium leprae.
MATERIALS AND METHODS
All individuals reported were registered patients of the Hansen's Disease Clinic of the LAC/USC Medical Center, Los Angeles, California, U.S.A. Patients were classified according to the criteria and nomenclature described by Ridley (12) and his associates. The classifications given in this paper were those initially made.
All tissue specimens were routinely processed for paraffin embedding and staining with hematoxylin and eosin (H&E) as well as with carbolfuchsin using the Fite-Farraco modification of the Ziehl-Neelsen method. The biopsy bacterial index (BI) is reported as a logarithmic number, the average of five randomly selected oil emersion fields. Because of extensive damage to the stores of tissue blocks and tissue sections in glass, caused by the 1994 Northridge, California, earthquake, not every biopsy taken at this medical center was available for review. Some histologic changes were graded semi-quantitatively as absent or negative (-), suggestive but insufficient for making a diagnosis or judgment (±), definitely present (+), and well-developed (++).
The diagnosis of a DTH reaction was considered to be primarily clinical, and its management was based upon the clinical findings. Skin biopsy was performed routinely. The historical information arousing suspicion of a DTH reaction at the time of the initial visit included: sudden worsening, the presence of progressive lesions of only a few months' duration, or the abrupt onset of painful nerves, nerve palsies or paresthesias. The physical findings arousing suspicion of a DTH reaction on the initial visits included: the presence of edema of an extremity or on the face, tumid or edematous lesions, especially with a purple color or a purplish cast, or tender nerves. In some patients, the historical and physical data were so striking as to permit a diagnosis of a DTH reaction at the time of the initial visit. Otherwise, observed clinical progression of the patient's condition in 1 or 2 weeks' time or important histologic findings, such as edema or epithelioid cell differentiation of macrophages in association with a high BI (11,13) were necessary before concluding with confidence that the patient had presented with a DTH reaction. The development of a DTH reaction in a treated, previously stable patient was based upon observing characteristic clinical worsening, and a biopsy which excluded bacteriological relapse or the presence of ENL and when compared with the original pretreatment biopsy almost always showed changes associated with DTH reactions.
The diagnosis of ENL, when considered, was usually easy, being characteristic both clinically (10) and histologically (8, 17). Microscopically, following Ridley, et al. (14), the ENL specimens were classified into three subgroups according to the primary site of inflammation, to wit, classical (subcutis or deep dermis), deep and superficial dermis, and superficial. Briefly, the classical pattern has little or no inflammatory infiltration in the papillary dermis but, with progression through the dermis, the infiltrate becomes more dense, peaking in the deep dermis or subcutis, producing a gradient of severity, so to speak. The superficial and deep pattern has equally dense focal inflammatory infiltrates distributed throughout the dermis, including its papillary portion, i.e., no gradient. The superficial pattern has dense infiltrates in the upper dermis only and is apt to be associated with necrosis. These three subgroups are comparable regarding the extent of neutrophil and lymphocyte infiltration, BI, etc. In the DTH to ENL patients, a pattern was said to be classical if a gradient of greater severity in the deep dermis or subcutis was present, even if the papillary inflammatory infiltrate was considered to be "significant" (8). Thus, in the DTH to ENL group, the prevalence of the classical pattern may be overestimated and that of superficial and deep, underestimated.
The few patients who were in reaction but for whom a precise designation as to type was not possible, even after repeated clinical and histological examinations, were not included in this study.
Two groups of DTH patients were controls. One consisted of seven patients who presented with a DTH reaction, were multibacillary, but did not develop ENL. The other consisted of 15 patients who presented as untreated, multibacillary, and nonreactional, but who developed a DTH reaction without subsequent ENL.
Two groups of ENL patients served as controls. One, a clinical control, consisted of 21 consecutive, untreated multibacillary patients who eventually developed ENL without a previous DTH reaction or who presented with ENL. The other, a histologic control, consisted of 33 randomly selected individuals who developed ENL before or after chemotherapy, who had biopsies of ENL lesions available for review, who had not been included in a previous histologic study (14), and were without a prior DTH reaction.
The M. leprae used in the lymphocyte transformation tests (LTT) were obtained from R.J.W. Rees (London, U.K.) and prepared by probe sonication (9). The level of endotoxin in this whole (soluble and particulate elements) sonicate preparation was less than 0.1 ng/ml with a Limulits amebocyte lysate assay (Whitaker Bioproducts, Walkersville, Maryland, U.S.A.) (9). A stimulation index of 3 or greater (the average of three replicates) was considered to be positive. The LTT controls consisted of five nonreactional BL patients treated for at least 10 years and six patients who had had ENL, but no DTH reactions, who had been under treatment for 10 to 25 years, median 13 years.
With very few exceptions, our patients are managed as outpatients. Consequently, in patients with ENL, to avoid excessive sedation, we do not exceed 200 mg of thalidomide daily at bedtime. If needed, rather than increase the dose of thalidomide beyond 200 mg daily to achieve an adequate remission, corticosteroids are used as adjunctive therapy. When initiating corticosteroid therapy for a DTH reaction, we anticipate their use for at least 6 months.
RESULTS
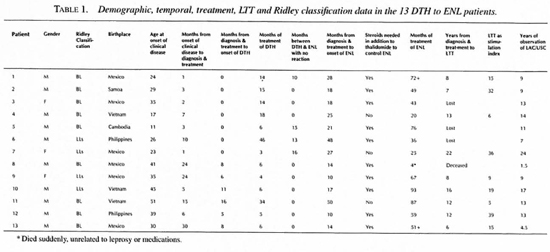
During a period of two decades we have observed 13 patients who, following a DTH reaction, developed ENL, the DTH to ENL group. Table 1 summarizes the demographic, temporal and LTT data as well as their initial classification. Nine of the patients had pretreatment biopsies and all of their therapy in our clinic. One patient, Case 1, had received rifampin for 4 weeks before being seen in our clinic. Another, Case 6, became a patient in our clinic 1 year after diagnosis and treatment but excellent records and early biopsies were made available for review. Case 7 had been taking dapsone for 4 months prior to clinic registration. A fourth. Case 13, had been taking dapsone and rifampin for 11 months before clinic registration and had an undiagnosed and untreated DTH reaction for the last three of these months. Compliance, as judged by faithfulness in keeping clinic appointments, was good in 12 of the 13, but only fair in Case 7. Cases 1-7 presented with DTH reactions; Cases 8-13 were not reactional at the time of initial presentation.
The DTH to ENL group was composed of 10 men and three women, not significantly different from the 2 to 1 ratio anticipated in multibacillary leprosy. The national origins of the DTH to ENL group proportionately reflected that of our entire clinic population. Nine were classified as BL and four as LLs. Only four patients had a reaction-free interval between termination of therapy for the DTH reactions and the onset of ENL. In nine, ENL had its onset while the patients were receiving prednisone for treatment of the DTH reaction.
During this same period of time we have observed only three patients with ENL preceding a DTH reaction. All were Mexican-born men (LLs) who experienced ENL when treated with dapsone alone and following bacteriological relapses had DTH reactions, one after the initiation of rifampin and minocycline, the other two presenting with a DTH reaction after years of poor compliance with antimicrobial treatment. The binomial probability (p value <0.05) indicates that the distribution of 13 patients with DTH to ENL and three patients with ENL to DTH is unlikely to be due to chance.
Among the DTH to ENL group, both clinical and histological aspects of their ENL differed importantly from the two ENL control groups. Clinically, in the DTH to ENL group, 10 of the 13 required prednisone in addition to thalidomide to obtain a satisfactory remission. This was in contrast to only two of 21 patients in the clinical ENL control group requiring supplemental prednisone. This difference was statistically significant (p value <0.001 by chi-squared test with the Yates' correction).
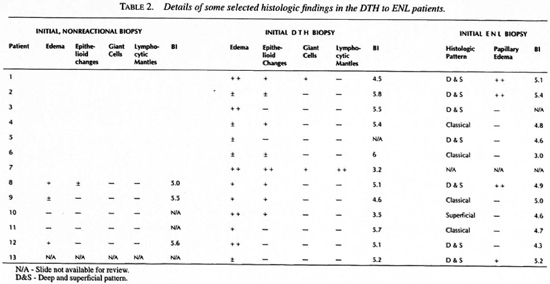
Table 2 summarizes some of the histologic changes found in the DTH to ENL group. The classical ENL histologic pattern was present in only four of the 12 specimens available for review. In contrast, in the histologic ENL control group the classical pattern was present in 29 of 33 (p value <0.005 by chi-squared analysis). Of the nonclassical patterns in the DTH to ENL group one was superficial and seven superficial and deep, four of the latter having marked edema of the papillary dermis.
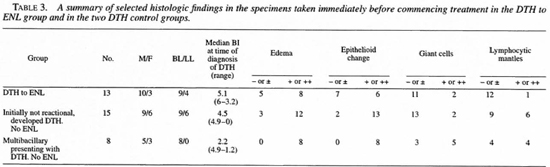
Among the DTH to ENL group, the histologic and microbiologic changes differed importantly from the two DTH control groups. Table 2 details DTH reaction-associated histologic changes in the DTH to ENL group and Table 3 compares the DTH reaction-associated histologic changes in the DTH to ENL group and in the two DTH reaction control groups. With the exception of giant cell formation, which was well developed in only one of the control groups, DTH reaction-associated changes were less well developed in the DTH to ENL group than in either of the two control groups. In four cases (2, 5, 6 and 13) even with benefit of hindsight, definite histologic evidence of a DTH reaction was not present. However, none of the control specimens were so lacking in corroboration of the clinical diagnosis.
At the time of onset of the DTH reaction, the DTH to ENL group had a heavier bacillary load than the controls. Figure 1 plots the Bis from biopsies obtained at the time of diagnosis of the DTH reaction in the DTH to ENL group and in the two DTH control groups. A BI of 5 or greater was present in 67% of the DTH to ENL group, in none of the multibacillary patients who presented with a DTH reaction but did not develop ENL, and in only 7% of those multibacillary patients who were initially not in reaction and who did not develop ENL. Statistically, the DTH to ENL group had significantly more lesional bacilli than did the two control groups (p = 0.007 and p = 0.02, respectively, using a T-test for independent samples).
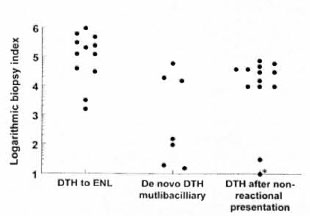
Fig. 1. Biopsy BI in specimens obtained immediately before starting treatment for a DTH reaction in the DTH to ENL group and the two control group. * = a BI of 0.
Among the DTH to ENL group, their LTTs in response to M. leprae challenge differed importantly from those of the two control groups (Fig. 2). Nine of the nine tested responded positively, but none of six ENL controls did so and only one of the five BL controls responded. In contrast, the LLT responses to challenge with interleukin-2 were similar in all three groups (data not shown). Case 1 is considered to be exemplary of the patients presenting with a DTH reaction who subsequently developed ENL.
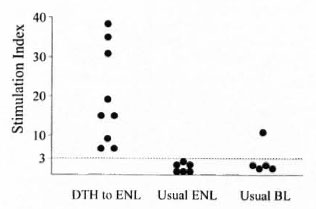
Fig. 2. Stimulation indexes from M. leprae sonicate-stimulated LTTs in 9 of 9 DTH to ENL patients tested, 6 long-term-treated post-ENL patients, and 5 long-term-treated BL patients.
Case 1. In May of 1989, a 24-year-old, Mexican-born man presented because of swelling of the face and hands for 1 month and a progressive skin eruption for 3 months. Four weeks before presentation, a physician in Tijuana, Mexico, prescribed rifampin 600 mg daily, which had no evident influence upon the progression of the patient's swelling or his eruption.
There was prominent, symmetrical, nonpitting, periorbital edema as well as edema elsewhere on his face and in both hands. An extensive, annular and confluent, erythematous and slightly indurated eruption was present on the trunk and on all four extremities. Both the inner and outer borders of the annuli were sharply marginated. In many of the lesions, the patient could not distinguish between pinprick and blunt stimulation. The lesions were not tender. There were no enlarged nerves.
Routine laboratory studies were normal, including leukocyte numbers and distribution, hemoglobin, hematocrit, urinalysis, serum globulin, serum chemistry panel and chest X-ray. The histology of the annulus of a skin lesion was characterized by a granulomatous infiltrate and numerous acid-fast bacilli (AFB) (BI of 4.5) and globi. The granulomas had slight epithelioid cell differentiation of macrophages, immature giant cell formation, edema, globi, but only sparse lymphocytes, a few plasma cells and no neutrophils (Fig. 3a). The initial impression was borderline lepromatous (BL) leprosy with a DTH reaction.
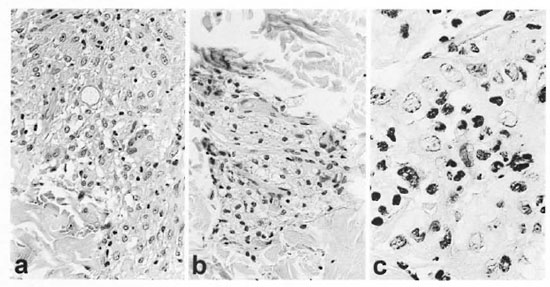
Fig. 3. Case 1. a = Initial biopsy showing a well developed giant cell, some epithelioid change, a Q-shaped globus in its central portion and appreciable edema (+) below the globus (H&E x40). b = Large foamy macrophages with a sprinkling of lymphocytes from clinically normal skin after prednisone was discontinued and before ENL developed (H&E x40). c = ENL, lesion prior to thalidomide or prednisone treatment showing neutrophils and other inflammatory cells infiltrating macrophages adjacent to eccrine glands (H&E x100).
Dapsone 100 mg daily and prednisone 40 mg daily were added to the rifampin 600 mg daily, a regimen associated with a gradual defervescence of both edema and skin lesions. The prednisone was slowly tapered and discontinued in July of 1990. In October of 1990, a biopsy of clinically normal skin from the lateral aspect of the upper portion of the left arm showed aggregates of large foamy macrophages in the papillary dermis and about appendages (Fig. 3b).
In May of 1991, the patient began to develop clinically typical ENL as judged by crops of painful and tender, dermal and subcutaneous, bright pink nodules on both upper and lower extremities in association with chills, fever, leukocytosis and a falling hematocrit (40.9 to 33.3). Microscopically, an ENL lesion showed the characteristic infiltration of neutrophils (Fig. 3c). Clinically, the ENL responded favorably but incompletely to thalidomide 200 mg at bedtime, but remitted completely with the addition of prednisone 30 mg daily. By August of 1991, prednisone was reduced to 30 mg every other day and, with progressively smaller doses, was discontinued in August of 1992. Thalidomide was reduced to 100 mg daily in November of 1991. Because of recurrent episodes of ENL, the patient was still receiving thalidomide 100 mg every other day, if needed, in July of 1997. Peripheral blood mononuclear cells (PBMC) obtained in July of 1997 challenged with M. leprae sonicate had a stimulation index of 15.
Case 9 is exemplary of patients who present without reaction but subsequently have DTH and ENL reactions sequentially.
Case 9. In March of 1989, a 37-year-old, Mexican-born woman presented with a 2-year history consistent with leprosy, including nasal stuffiness, numbness in the hands and feet, thinning of the eyebrows and a progressive asymptomatic skin eruption. Physical changes included widely distributed erythematous, poorly defined, digitate lesions, and an extensive decrease in pinprick perception. A routine blood count and blood chemistries were normal. A skin biopsy showed perivascular and peri-appendageal accumulations of foamy histiocytes with few lymphocytes (Fig. 4a), the epidermis was normal and a Grenz zone evident; a Fite-Farraco stain demonstrated numerous AFB and globi, BI of 5.5. The patient was classified as LLs.
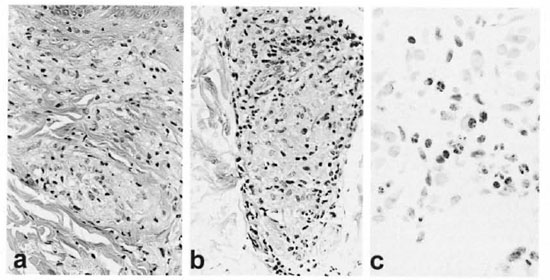
Fig. 4. Case 9. a = Initial biopsy showing aggregates of foamy macrophages in papillary and upper reticular dermis and a sparse lyinphocytic infiltrate (H&E x40). b = DTH reaction before prednisone showing epithelioid cell change, some sequestration of lymphocytes to the periphery of the epithelioid cell aggregate and a suggestion of edema at lower portion of the granuloma (H&E x40). c = Neutrophils and lymphocytes in an ENL lesion infiltrating macrophages near the junction of the dermis and subcutis (H&E x40).
Initially, the patient received minocycline 100 mg daily for 12 weeks as a participant in a clinical trial, as published elsewhere (4). At the end of the trial, she was started on dapsone 100 mg daily and rifampin 600 mg daily. In the middle of September 1989 her skin lesions, which had been regressing, became more prominent. In early October, with the clinical impression of a DTH reaction, a biopsy demonstrated edema and some epithelioid change (Fig. 4b) and prednisone was started at 40 mg daily. By early December 1989, she was receiving 30 mg of prednisone every other day and was considered to be doing well. In mid-December, the patient developed ENL as judged clinically by red bumps, chills, fever and malaise, and microscopically by characteristic infiltration of neutrophils (Fig. 4c). Because the patient had never been pregnant and was understandably reluctant to have tubal ligation, thalidomide could not be used. From January of 1990 through May of 1993, satisfactory control of the ENL could not be achieved with prednisone doses of rarely less than 40 mg daily and often higher, and often supplemented with intramuscular triamcinolone as well as clofazimine in doses of 200 mg daily. Serious complications included diabetes mellitus and thrombophlebitis with pulmonary emboli. Inpatient care at the Gillis W. Long Hansen's Disease Center, Carville, Louisiana, U.S.A., established that her ENL was thalidomide responsive. After 3½ years of virtually unremitting ENL and poor control with prednisone, the patient elected to have a tubal ligation in order to make her eligible to receive thalidomide. Initially, she responded well to thalidomide at 100 mg nightly in association with a slow taper of the prednisone taken every other day. At lower doses of prednisone, 200 mg of thalidomide daily was necessary for a 3-month period. The patient then did well on 100 mg thalidomide nightly until its daily use was discontinued without incident in August of 1996. PBMC obtained in the spring of 1997 challenged with M. leprae sonicate had a stimulation index of 9.
DISCUSSION
Taken as a group, the 13 patients who developed DTH reactions followed by ENL appear to be distinctive as judged by a number of features.
Clinically, the ENL in the DTH to ENL group had a significantly larger number of patients requiring supplemental prednisone in addition to thalidomide than did the control group of 21 consecutive patients with ENL. Microscopically, the ENL in the DTH to ENL group had significantly fewer patients with the classical histologic pattern than did the control group of 33 randomly selected ENL patients. Microscopically, the DTH-associated changes were less well developed in the DTH to ENL group than they were in either of the two DTH control groups. Bacteriologically, at the time of diagnosis of the DTH reaction, the lesions in the DTH to ENL group had significantly more bacilli than did those in the two control groups. Finally, after the reactional states were in complete remission, M. leprae -sonicate stimulated LTTs in the DTH to ENL group were positive in all of those tested, clearly a different result than was found in long-term-treated BL or ENL patients without preceding DTH reactions.
Could the DTH to ENL group be attributable to chance alone? Because in our clinic population, which has a high incidence and prevalence of both DTH and ENL reactions, consistent with those reported in recent epidemiological studies (1, 3, 7, 17, 18), one might expect a small number of patients to have both reactions. However, if a chance matter, one would expect ENL to precede DTH reactions as often as it follows a DTH reaction and this has not been the case, an apparently significant difference (p value <0.05). Furthermore, if a matter of chance, one would not anticipate the observed histologic segregation, i.e., the low prevalence of a classical histologic pattern in the DTH to ENL group, nor the therapeutic difference, i.e., the poorer response to thalidomide in the DTH to ENL group. Moreover, if a matter of chance, one would not predict the significantly larger numbers of bacilli in the DTH to ENL group. Finally, the three ENL to DTH patients have been associated with bacteriological relapses, ENL before relapse, and DTH after relapse, a pattern consistent with some previous reports(5, 20).
We originally thought that individuals having the DTH to ENL sequence of reactions had DTH reactions that were downgrading, i.e., a move toward the lepromatous end of the spectrum. That this did happen seems evident, DTH reactions being characterized by a Type 1 cytokine profile but ENL Type 2 (22), but that this was not a permanent change is also equally clear, judging from the LTT results.
The DTH to ENL group shares some features with those LL patients who relapsed as BT, in particular, the shifting host immunologic postures (5, 20) and the evident restoration of lepromin reactivity in some (20). Also, of the eight LL patients who relapsed as BT, reactions were common, there being four with DTH and five with ENL reactions. However, the absence of bacteriological relapse in the DTH to ENL group separates the DTH to ENL patients from those LL patients relapsing as BT.
Our observed histologic changes in DTH reactions are consistent with previous reports. Ridley (11) emphasized edema as the most constant finding but also described epithelioid cell differentiation and giant cell formation, both foreign body and Langhans' types, as well as necrosis, if severe. Also, he recognized that in some cases there may be little or no histologic findings of a DTH reaction. In a detailed study, Ridley and Radia (13) followed serial biopsies to outcome in 12 patients selected for study because of the good correlation between clinical and histologic evidence of a DTH reaction. Their two patients who downgraded and their two who had not changed granulomatous posture had changes similar to those in our DTH to ENL group, i.e., comparatively poorly developed histologic changes and little, if any, bacteriolysis.
Concerning ENL histologic subtypes, Ridley, et al. (14) found that the prevalent primary site of inflammation varied with the patient's birthplace, i.e., 83% classical in Mexican-bom but only 8% classical in Malaysian-born. Among the 13 DTH to ENL patients were six Mexican-bom subjects and, of these, four of the five (80%) specimens available for review had a superficial and deep pattern, indicating that preponderance of the superficial and deep subtype is truly a characteristic of the DTH to ENL group and not an artifact of the patient's birthplace.
The oscillations in host immunologic postures in the DTH to ENL group are striking. First comes a period of apparent tolerance when, clinically or subclinically, the host tolerates abundant proliferation of M. leprae, previously associated with a Type 2 cytokine profile (21), followed by a reversal reaction associated with a Type 1 profile (21), followed by ENL associated with another Type 2 profile and, finally, followed by quiescence but with LTTs indicating a likely Type 1 profile if the host were further challenged by M. leprae.
These labile responses are difficult to explain. They do not appear to be a trait of a subset of patients identified by national origin, age, gender or granulomatous posture. Shifts in the dominant antigen recognized is a possibility which should be investigated, but for which there is no relevant evidence. Because the host's adoptive immune cytokine profile may be determined by the cytokines elaborated by cells of the native (nonadoptive) immune response, shifts in signals from the native immunocytes might change the cytokine pattern of the adoptive response (15). That such is possible is suggested by the observed precipitation of ENL by the parenteral administration of recombinant human interferon gamma (16). A recent report on lesion-derived T-cell clones as to frequency of Types 1, 2 and 0 cytokine profiles is also consistent with changing signals leading to changing T-cell cytokine repertoire (19). That is to say, because most M. Leprae -responsive, BL lesion-derived clones were Type 0, this suggests a pluripotential T-cell reservoir able to mediate a variety of host responses as well as various cytokine profiles.
This immunologically labile subset of patients merits careful scrutiny, perhaps helping to solve some of the problems surrounding the immunopathogenesis of DTH and ENL reactions, as well as that of the granulomatous spectrum.
Acknowledgment. Dr. Wayne Meyers of the Armed Forces Institute of Pathology and Dr. David Scollard of the Gillis W. Long National Hansen´s Disease Center provided histologic slides from biopsies of patient number 6. Dr. Leo Yoder of the Gillis W. Long National Hansen's Disease Center provided the case-records of patient number 6. Ms. Annie Hartono and Ms. Annali/a Legaspi. UCLA Division of Dermatology, gave expert technical assistance and Ms. Min Xiang, USC Biometry, expert statistical assistance.
REFERENCES
1. BECX-BLEUMINK, M . and BERHE, D. Occurrence of reactions, their diagnosis and management in leprosy patients treated with multidrug therapy: experience in the Leprosy Control Program of the All Africa Leprosy and Rehabilitation Training Center (ALERT) in Ethiopia. Int. J. Lepr. 60 (1992) 173-184.
2. BERNINK, E. H. M. and VOSKENS, J. E. J. Study on the detection of leprosy reactions and the effect of prednisone on various nerves, Indonesia. Lepr. Rev. 68(1997) 225-232.
3. BWIRE, R. and KAWUMA, H. J. S. Hospital-based epidemiological study of reactions, Buluba Hospital, 1985-89. Lepr. Rev. 64 (1993) 325-329.
4. GELBER, R. H., FUKUDA, K., BYRD. S., MURRY, L. P., SUI, P., TANG, M. and REA, T. H. A clinical trial of minocycline in lepromatous leprosy. Br. Med. J. 340(1992)91-92.
5. GlRDHAR, B. K.. GlRDHAR, A., CHAUHAN, S. L., MAI.AVIYA, G. N., HUSAIN, S. and MUKHERJEE, A. Borderline-tuberculoid relapse in lepromatous leprosy. Lepr. Rev. 64 (1993) 157-163.
6. Job. C. K., JACOBSON, R. R. and HASTINGS, R. C. Simultaneous upgrading reaction and erythema nodosum leprosum in a patient with lepromatous leprosy. Int. J. Lepr. 56 (1988) 437-442.
7. LOCKWOOD, D. N. J., VlNAYAKUMAR, S., STANLEY, J. N. A.. McADAM, K. P. W. J. and COLSTON, M. J. Clinical features and outcome of reversal (type 1) reactions in Hyderabad, India. Int. J. Lepr. 61 (1993)8-15.
8. MABALAY, M. C, HELWIG, E. B., TOI.ENTINO, J. G. and BINFORD, C. H. The histopathology and histochemistry of erythema nodosum leprosum. Int. J. Lepr. 33(1965)28-49.
9. MEHRA, V, BLOOM, B. R., TORIGIAN, V. K., DANIZA, M., REICHEL, M., YOUNG, S. M. M., SALGAME, P., CONVIT, J., HUNTER, S. W., MCNIEL, M.. BRENNAN. P. J.. REA, T. H. and MODLIN. R. L. Characterization of Mycobacterium leprae cell wall-associated proteins with the use of T lymphocyte clones. J. Immunol. 142 (1989) 2873- 2878.
10. REA, T. H. and LEVAN, N. E. Erythema nodosum leprosum in a general hospital. Arch. Dermatol. 111 (1975) 1575-1580.
11. RIDLEY, D. S. Reactions in leprosy. Lepr. Rev. 40 (1969)77-81.
12. RIDLEY, D. S. Histological classification and the immunological spectrum of leprosy. Bull. WHO 51 (1974) 451-465.
13. RIDLEY, D. S. and RADIA, K. B. The histological course of reactions in borderline leprosy and their outcome. Int. J. Lepr. 49 (1981) 383-392.
14. RIDLEY, D. S., REA, T. H. and McADAM, K. P. W. J. The histology of erythema nodosum leprosum. Varient forms in New Guineans and other ethnic-groups. Lepr. Rev. 52 ( 1981 ) 65-78.
15. ROMAGNANI, S. Th1 and Th2 in human disease. Clin. Immunol. Immunopathol. 80(1996) 225-235.
16. SAMPAIO, E. P., MOREIRA, A. L., SARNO, E. N., MALTA, A. M. and KAPLAN, G. Prolonged treatment with recombinant interferon γ induces erythema nodosum leprosum in lepromatous leprosy patients. J. Exp. Med. 175 (1992) 1729-1737.
17. SCOLLARD, D. M., SMITH, T, BHOOPAT, L., THEETRANONT, C, RANGDAENG, S. and MORENS, D. M. Epidemiologic characteristics of leprosy reactions. Int. J. Lepr. 62 (1994) 559-566.
18. VAN BRAKEL. W. H.. KHAWAS, I. B. and LUCAS, S. B. Reactions in leprosy: an epidemiological study of 386 patients in west Nepal. Lepr. Rev. 65 (1994) 190-203.
19. VERHAGEN, C. E., WIERENGA, E. A., BUFFING, A. A. M., CHAND, M. A., FABER, W. R. and DAS, P. K. Reversal reaction in borderline leprosy is associated with a polarized shift to type 1-like Mycobacterium leprae T-cell reactivity in lesional skin. J. Immunol. 159 (1997) 4474-4483.
20. WATERS. M. F. R. and RIDLEY, D. S. Tuberculoid relapse in lepromatous leprosy. Lepr. Rev. 61 (1990) 353-365.
21. YAMAMLRA. M., UYEMURA. K.. DEANS, R. J., WEINBERG, K., REA, T. H.. BLOOM. B. R. and MODLIN, R. L. Defining protective responses to infectious pathogens: cytokine profiles in leprosy lesions. Science 254 (1991) 277-279.
22. YAMAMURA, M., WANG, X.-H., OHMEN, J. D., UYEMURA, K., REA, T. H., BLOOM, B. R. and MODLIN, R. L. Cytokine patterns of immunologically mediated tissue damage. J. Immunol. 149 (1992) 1470-1475.
1. M.D., Division of Dermatology. University of Southern California School of Medicine. Los Angeles. California 90033. U.S.A.
2. Ph.D., Division of Dermatology. University of California-Los Anizeles School of Medicine. Los Angeles, California 90095. U.S.A.
Reprint requests to: T. H. Rea, M.D.. Division of Dermatology. USC School of Medicine. 2025 Zonal Avenue, Los Angeles. CA 90022. U.S.A. e-mail: tea@hsc.usc.edu
Received for publication on 26 March 1998.
Accepted lor publication in revised form on 5 August 1998.