- Volume 66 , Number 3
- Page: 328–39
The nose in leprosy: immunohistology of the nasal mucosa
ABSTRACT
A detailed study of the nose was undertaken in 40 leprosy patients with different classifications of leprosy and different durations of disease at two hospitals in Brazil. This manuscript describes the immunohistochemical data on cellular infiltrates in the nasal biopsies of those patients. It was surprising that the damage to the whole depth of the nasal mucosa, epithelium and lamina propria was considerable, as was the case in the nasal mucosa which looked relatively normal during clinical inspection. The epithelium showed large holes which looked like very extended goblet cells. Very obvious was the lack of vasoconstriction after cocaine application, and the vessels also showed a lack of staining with factor VIII, possibly indicating a disruption of the endothelium. The number of neurofilaments was extensively reduced in all leprosy groups compared to normal controls. As in the skin, an increased number of CD68+ cells was found in the lamina propria of the nasal mucosa of the lepromatous patients. Contrary to findings in the skin, in the nasal mucosa of the borderline/lepromatous patients the number of CD4+ cells was increased and the number of CD8+ cells was decreased compared to normal controls. The number of CD8+ cells tended to be more reduced when the history of leprosy was longer. It is not clear as yet whether the reduced numbers of CD8+ cells are acquired during infection or whether persons with a low number of CD8+ cells in the nose might have a higher risk of acquiring leprosy.RÉSUMÉ
Une étude détaillée des lésions nasales a été enterprise chez. 40 patients issus de deux hôpitaux brésiliens et atteints de lèpre de durée d'évolution et de classification variable. Cet article décrit les données inimuno histochimiqu.es des infiltrats inflammatoires observés à partir des biopsies nasales de ces patients. Il fut surprenant de constater l'importance des lésions de la muqueuse, à la fois de I'épithélium et de la lamina propria, même lorsque la muqueuse nasale semblait relativement normale à l'inspection clinique. L'epithelium montrait de grands trous qui ressemblaient à des cellules à mucus hypertrophiées. Il y avait une absence très évident de vasoconstriction après l'administration locale de cocaine, et les vaissaux montraient une absence de marquage par le facteur VIII, suggérant une atteinte de l'endothélium. Le nombre de neurolilments était nettement rtkliut dans chaque groupe atteint de lèpre, en comparaison de contrôles normaux. Comme dans la peau, la lamina propria de la muqueuse nasale des patients lépromateux avaient un nombre important de cellules CD68+. A l'opposé, contrastant avec les données cutanées, un nombre plus important de cellules CD4+ et un nombre moins important de cellules CD8+ par rapport aux contrôles, étaient présents dans la muqueuse nasale des patients borderline/lépromateux. Le nombre de cellules CD8+ aviat tendance à être d'autant plus réduit que la durée d'évolution de la lèpre était longue. Il reste cependant à determiner si le nombre réduit de cellules CDS+ est acquis en cours d'infection ou si les individus avec un nombre réduit de cellules CD8+ dans le nez one un risque plus important de développer la lèpre.RESUMEN
Se realizó un estudio detallado de la nariz, de 40 paeientes con diferentes tipos de lepra y eon diferentes tiempos de evolución de la enfermedad en 2 hospitales de Brasil. En este manuscrito se describen los hallazgos inmunohistoquímieos de los infiltrados celulares en las biopsias nasales de los paciente. Fue sorprendente encontrar que el daño de toda la mucosa nasal, incluyendo el epitelio y la lámina propia, fue considerable, a pesar de que la mucosa pareció relativamente normal en la inspección clínica. El epitelio mostró hoyos grandes que pareceieron como células globosas muy extendidas. Fue muy obvia la falta de vasoconstricción después de la applicación de cocaína. Los vasos también mostraron la ausencia de tinción para factor VIII, quizá por la ruptura del endotelio. Todos los pacientes mostraron un número extremadamente reducido de neurofilanientos. Como en la peil, en la lámina propia de la mucosa nasal, los pacientes con lepra lepromatosa tuvieron números elevados de células CD68+. Comparados con los controles sanos, los pacientes lepromatosos y pre-lepromatosos tuvieron números elevados de células CD4+ y números reducidos de células CD8+; esto fue contrario a lo observado en la piel. El número de células CD8+ tendió a ser más reducido cuando la evolución de la enfermedad fue más larga. No está claro si los números reducidos de células CD8+ son el resultado de la infección o si las personas con números bajos de células CD8+ en la nariz tienen mayor riesgo de adquirir la enfermedad.In 1874 Armauer Hansen described the causative organism of leprosy, the acid-fast bacterium Mycobacterium leprae (6). From that time onward, leprosy had to be considered an infectious disease. However, it has not been possible to deliberately infect someone with leprosy. Although it has been reported that some individuals developed leprosy from being tattooed or from skinning or preparing armadillos for cooking (2, 25), the respiratory tract is proposed as the portal of entry, the nose playing a central role(2, 29).
Studies concerning the nose in leprosy are mainly aimed at the spreading of the disease via nasal secretion (7, 26). Only a few studies have focused on the inflammatory process of the nasal mucosa itself (1, 8, 9, 16), not only macroscopically investigated by an eye, nose and throat (ENT) examination but also looking at the cellular infiltration. In 1966 Job, et al. described the clinical and histopathological appearance of leprous rhinitis (9). In his group of 7500 patients he only observed septal perforations in lepromatous patients and never in the tuberculoid or intermediate forms. He divided the lepromatous type into four stages, from invasion of bacteria to the stage of resolution and fibrosis. In 48 biopsies from 38 patients with tuberculoid and intermediate leprosy, he found infiltration of the nasal mucosa with lymphocytes, epithelioid cells and giant cells, an increase in the number of mucus-producing cells in the epithelial lining, and a marked increase in vascularity.
McDougall, et al. took 138 biopsies from 4 borderline and 31 lepromatous patients before and after dapsone treatment (16'). They found no changes in cellular infiltrates and no evidence of leprosy infection in their borderline patients. In the lepromatous patients, they were struck by the frequency and numbers of bacilli within the endothelial lining cells of the blood and lymph vessels and the presence of bacilli free within the lumina of these vessels.
No studies, to our knowledge, have recently been performed with modern immunohistochemical methods to study cellular infiltration and the role of the nasal mucosa in the immune response of leprosy patients. Therefore, nasal biopsies from Brazilian leprosy patients with different classifications of leprosy and different durations of disease were studied immunohistochemically to further characterize the cellular infiltrates in the nasal mucosa.
MATERIALS AND METHODS
Patients
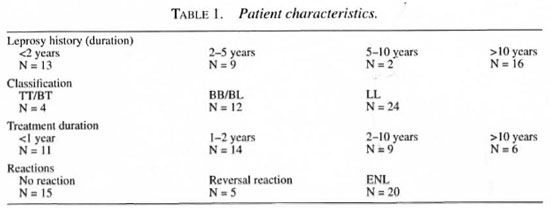
Forty Brazilian leprosy patients [34 men and 6 women, mean age 40 years (range 19-75)] with different classifications of leprosy and different durations of disease were recruited in leprosy centers in Bauru (Instituto Lauro de Souza Lima, 22 patients) and in Rio de Janeiro (Santa Cruz, 18 patients). The ethnic origin of the patients was 19 Latino, 15 Negroid and 6 Caucasian. Twenty-four patients were classified as lepromatous, 12 as borderline (10 BB, 2 BL) and 4 as tuberculoid (2 BT, 2 TT). Thirteen patients were known to have had leprosy for less than 2 years, 9 for 2-5 years, 2 patients for 5-10 years and 16 for more than 10 years. Eleven patients were treated for less than 1 year, 14 patients for 1-2 years, 9 patients for 2-10 years and 6 patients for more than 10 years. Twenty-nine patients received multidrug therapy (MDT) and 11 patients were receiving or had received monotherapy (Table 1). The patients were compared to 10 non-Brazilian Caucasian controls. The controls were healthy patients who underwent rhinoplastic surgery at the ENT department of the Erasmus University Medical Center, Rotterdam, The Netherlands. These patients showed no abnormalities of the nasal mucosa at rhinoscopy and nasal endoscopy, and had a normal sinus X-ray.
Study design
A standardized history was taken from all patients and a protocolized ENT examination was done. The standardized history was taken with the aid of a local physician who translated all the questions and answers. The questions were focused on the history, treatment, and symptoms of leprosy with special emphasis on ENT problems. The ENT examination consisted of inspection of the face, ears, internal and external nose, and the nasopharynx. A nasendoscopy, nasopharyngoscopy and laryngoscopy was performed on every patient using a Storz 30º and 70º nasendoscope and flexible laryngoscope.
Nasal biopsies
From 34 patients (4 tuberculoid, 10 borderline and 20 lepromatous) and 10 controls, biopsies of the nasal mucosa were taken from the lower edge of the inferior turbinate, about 2 cm posterior to the front edge, and sometimes from the nasal septum using a Gerritsma forceps with a cup diameter of 2.5 mm. Local anesthesia was obtained by placing a cotton-wool carrier with 50-100 mg cocaine and 3 drops of epinephrine (1:1000) under the inferior turbinate or against the septal mucosa without touching the place where the biopsy would be taken (5). The biopsy specimens were embedded in Tissue-Tek II O.C.T. compound in a gelatin capsule and immediately frozen in liquid nitrogen.
Staining procedure
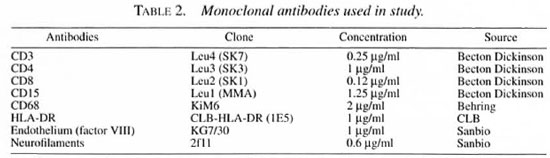
Staining was performed by means of the long-chain biotin streptavidine-alkaline phosphate method (supersensitive Bio-Genix; Klinipath, Duiven, The Netherlands). Each tissue specimen was cut into serial 6-µm-thick sections on a Reichert-Jung 2800e Frigocut cryostat and transferred to poly-L-lysine-coated microscope slides (Sigma, Bornem, Belgium), dried and stored at -80ºC for a maximum of 3 months. The specimens were raised to room temperature, dried and fixed in acetone for 10 min at room temperature.
The staining procedure was continued by placing the slides in a half-automatic stainer (Sequenza; Shandon Scientific, Zeist, The Netherlands). Following this, the sections were incubated for 10 min with 0.5%-1 % bovine serum albumin (BSA; Sigma) in phosphate-buffered saline (PBS). The sections were then incubated with normal goat serum (CLB, Amsterdam, The Netherlands) for 10 min and subsequently for 60 min with the relevant monoclonal antibody (Table 2). They were then rinsed with PBS for 5 min and incubated with Link (goat anti-mouse long-chain biotinylated supersensitive AP, BioGenex AZ000UM; Klinipath) for 30 min, rinsed with PBS for 5 min, incubated with Label (streptavidine-alkaline phosphatase, BioGenex AZ000UM; Klinipath) for 30 min. They were then rinsed once more in PBS for 5 min and TRIS buffer (0.1 M pH 8.5) for 5 min and incubated for 30 min with a New Fuchsin substrate (Chroma, Kongen, Germany). Finally, the sections were rinsed in distilled water, counterstained with Gill's hematoxylin and mounted in glycerin-gelatin.
Control staining was performed by substitution with PBS and incubation with an irrelevant monoclonal antibody of the same subclass. For the general description hematoxylin and eosin (H&E) staining was performed.
Wade-Fite staining. Wade-Fite staining was done according to the standard procedure (15).
Monoclonal antibodies
The monoclonal antibodies used are described in Table 2.
Light microscopic evaluation
The surface area of two entire sections, and of the epithelium and lamina propria separately, was measured using the Kontron Image Analysis System Videoplan 2.1. The number of positive cells per section was counted using a 400x magnification. The number of positive cells/mm2 was calculated. When cells could not be counted separately (neurofilament, endothelium, HLADR), the amount of positivity was scored semi-quantitatively by ranking all biopsies in ascending order and giving each a rank number. These numbers were then used for statistical analysis.
Statistical analysis
Immunohistological parameters were compared among the tuberculoid, borderline and lepromatous leprosy patients and compared to the normal controls. Also, the duration of the disease, expressed as the years of known leprosy by history, was analyzed.
Semi-quantitative measurements were put in ranking order. The rank number of a certain section was used for further statistical analysis.
Since the frequency distributions were not symmetrical and the variances were unequal, the Kruskal-Wallis one-way analysis of variances was used to calculate the overall p value. A p value of <0.05 was considered to indicate a significant difference between groups. When the Kruskal-Wallis analysis resulted in significant differences among the groups, the Wilcoxon rank sum test was used to further differentiate among the four different groups.
RESULTS
General symptoms and signs
More than half of the patients had signs and symptoms of the disease on the hands, feet and face; 18 patients had complaints about the shape of the ear and in 21 patients (two tuberculoid) deformities of the ear were found. Seventy percent of the borderline and lepromatous patients had complaints of the nose. Patients indicated nasal blockage (18/40) and crusts (25/40) as their major complaint, and 26 patients had changes of the external nose. In the lepromatous and borderline patients, endoscopic examination showed different stages of destruction from small ulceration of the mucoperichondrium to septal perforation and, finally, complete destruction of the cartilaginous and bony septum and even destruction of all the nasal turbinates in many lepromatous and borderline patients. None of the tuberculoid patients had abnormalities in the nose, but they showed a light color and some dryness of the nasal mucosa.
General histology
The sections of nasal mucosa had an average surface of 3 mm2. The general histology differed from normal, from partial epithelial disruption and changes in appearance of the vessels, to a totally distorted architecture in which hardly any normal structure could be recognized. The apparently normal biopsies usually showed a lining of ciliated columnar epithelium with or without goblet cells and of partially stratified cuboidal epithelium. All of the normal controls showed an intact or only minimally damaged epithelium. However, in the leprosy groups extensive damage of the epithelium was found in 50% of the tuberculoid, 60% of the borderline and 70% of the lepromatous patients. In practically all leprosy patients the epithelium showed large holes which appeared to be very enlarged goblet cells (Fig. la and b)
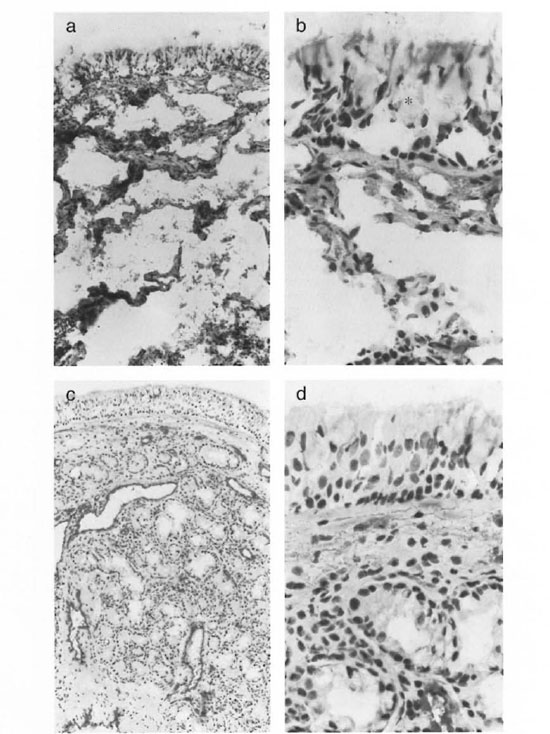
Fig. 1. Staining of the nasal mucosa with KG7/30 (factor VIII, staining the endothelium) of a lepromatous leprosy patient (a and b)) and a normal control (c and d). The epithelitim of the lepromatous leprosy patient showed large boles which appeatred to be very enlarged goblet cells (*). After vasoconstrictive cocaine application, normal biopsies showed extensive vasoconstriction in the subepithelial layer and obvious vasoconstriction in the lower parts of the lamina propria (c). In leprosy biopsies a marked decrease of vasoconstrietion was seen cornpared to the normal controls. Often the vessels were not constricted at all (a). The endothelium seemed to be lacking in the leprosy patients (b) (photomicrographs a and c x200; photomicrographs b and d x400).
The normal lamina propria usually consisted of a looser subepithelial cell-rich layer with most of the mucous glands and a deeper collagenous, cell-poor layer containing most of the vessels on to the bone. Normal biopsies (after vasoconstrictive cocaine application) showed extensive vasoconstriction in the subepithelial layer and obvious vasoconstriction in the lower parts of the lamina propria (Fig. 1c and d). However, in the leprosy biopsies a marked decrease of this vasoconstriction was seen; often the vessels were not constricted at all (Fig. la).
Bacillary index (BI)
In 18 patients M. leprae were detected using the Wade-Fite staining. Of these 18 patients half scored more than 10 bacilli per microscopic field.
Immunohistological staining
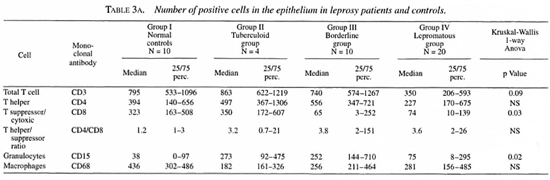
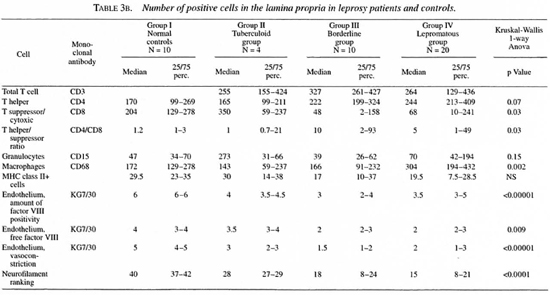
The median number and range of cells and the p values are indicated in Tables 3 and 4.
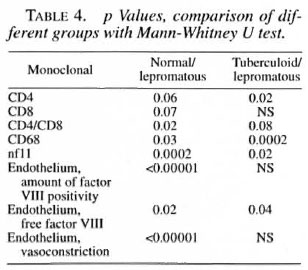
T cells. T cells were stained for CD3, CD4 and CD8 positivity. In the epithelium the total T cell numbers/mm2 tended to be lower in the lepromatous group compared to the normal controls and the other two groups. The number of CD8+ cells was normal in the tuberculoid group but significantly reduced in both of the other groups. No significant differences were found in the CD4+ population.
In the lamina propria, however, the number of CD4+ cells tended to be higher in the borderline and lepromatous groups compared to the controls (p = 0.07). The number of CD4+ cells was significantly higher in the lepromatous groups than in the tuberculoid groups. The number of CD8+ cells was significantly lower in the borderline and lepromatous groups compared to the controls. Hence, the CD4/CD8 ratio in the lamina propria was significantly increased in the borderline and lepromatous groups. Also, CD8+ cell numbers tended to be more reduced when the history (defined as the number of years which had passed since the leprosy was diagnosed) was longer. This was more obvious in the borderline group than in the lepromatous group (data not shown).
Granulocytes. CD 15+ cells in the epithelium were significantly increased in all leprosy groups compared to the controls. No differences were found in the lamina propria.
Macrophages. A significantly lower number of CD68+ cells was found in the lamina propria of the tuberculoid group compared to all other groups. The number of CD68+ cells in the lamina propria was significantly higher in the lepromatous groups compared to the controls (p = 0.03) and to the tuberculoid group (p = 0.0002). No differences were found in the epithelium.
HLA-DR+ cells. The number of HLA-DR+ cells was determined semi-quantitatively. The degree of positivity varied considerably. In most of the biopsy specimens the cells of the mono-histiocytic lineage and endothelium were positive. When more positivity occurred, mononuclear cells, i.e., activated T and B cells, were found to be positive. Sometimes the cytoplasm of the epithelium (including that of the mucous glands) became positive.
Endothelial cells. In the biopsies of the normal controls, vessels usually stained dark red in a light rose background due to free factor VIII in the tissues (Fig. lc and d). The vessels, which appeared to be well constricted, were stained all around the surface (Fig. lc). In the leprosy biopsies, a marked reduction in vasoconstriction was seen. Also apparently less free factor VIII was seen in the tissue. The vessels usually were stained only partly along the walls. At these sites the endothelium seemed to be lacking (Fig. la and b).
Neurofilaments. Neurofilaments were only found in the lamina propria. A significant reduction of the amount of positivity was found in all of the leprosy groups compared to the normal controls. Also, the amount of positivity in the lepromatous group was significantly reduced compared to the tuberculoid group. A significant inverse correlation was found between the amount of neurofilaments and the existence of septal perforations (p = 0.02).
DISCUSSION
In this study clinical data concerning the nose and data on immunohistochemical findings in the nasal mucosa of leprosy patients and normal controls are compared. Immunohistochemical investigations have frequently been performed on the skin of leprosy patients (19, 20 33, 36, 39). However, to our knowledge this study is the first to describe antigenic characteristics of cells in the nasal mucosa.
Some leprologists do not consider the skin to be of great importance as the portal of entry for M. leprae, although some still do (2, 14, 23). Most consider leprosy to be an airborne disease like tuberculosis (30), in which infectious patients discharge bacteria from their nasal mucosa (27, 30, 31). The respiratory tract is proposed as the portal of entry, the nose playing a central role (2, 29).
However, it is still not known why some individuals develop leprosy and others do not. An innate immunity is proposed for some individuals (2, 22, 40) but in the majority of infected persons the cell-mediated immunity (CMI) seems to be of crucial importance(21). For a short period of time it was thought that the HLA locus would be the decisive factor (3), but this was soon refuted (35). However, it was later shown that the HLA-DR phenotype had some influence on the type of leprosy which developed after infection (24, 38).
Stoner, et al. suggested that the way of entry of M. leprae antigens into the immune system could be of importance, supported by the concept of a peripheral and a central lymphatic compartment (35). Their theories were later expanded by Naafs (22) and Das and Naafs (3) who theorized that a balance between immune tolerance induced via the central lymphatic compartment, sensitized via a respiratory portal of entry, and delayed-type hypersensitivity, induced via the peripheral lymphatic compartment, sensitized by the introduction of bacilli through the skin (scratching), determines the outcome of the infection. Recently, it has been suggested that the antigens presented via the nasal mucosa could suppress the CMI (12). Whether the differences in the CD4/CD8 ratio or the changes in the TH1/TH2 ratio (17) are the most important has not yet been determined. Klatser, et al. have shown by means of the polymerase chain reaction (PCR) that even healthy contacts in an endemic environment have M. leprae DNA in their nasal mucosa (11) and that visitors to a leprosy hospital have transient positive nose swabs as well.
In 1966 Job, et al. described the histology of the nasal mucosa of leprosy patients and differences in the cellular infiltrates (9). However, at that time further characterization of the infiltrates was not possible.
In this study, it was surprising that the damage to the whole depth of the nasal mucosa, epithelium and lamina propria was considerable, even in nasal mucosa which looked relatively normal during clinical inspection. In tuberculoid patients, who macroscopically did not show any damage of the nasal mucosa, histopathologically extensive damage of the epithelium was found in half of them.
The epithelium showed large holes which looked like very extended goblet cells. This phenomenon was also described by Job, et al. (9). A possible explanation for this could be that due to extensive production of mucus stimulated by the continued bacterial superinfections, the goblet cells become overextended. On the other hand, the seromucous glands of the leprosy patients were significantly reduced compared to the normal controls and were surrounded by inflammatory cells. It could well be that a shift has taken place from production of mucus in the glands to production in the goblet cells, resulting in a change in mucus constituents and, hence, to a lack of mucosal defense.
Very obvious was the lack of vasoconstriction after cocaine application, which was already encountered when taking the biopsies. Although our group is very experienced in taking biopsies in all sorts of nasal mucosal disease (5), having generally less than 2‰ epistaxis, a considerable number of the leprosy patients showed some epistaxis after the biopsy. A few even had significant epistaxis. Also, in the factor VIII staining of the endothelium an apparent lack of vasoconstriction was found. Moreover, in the leprosy biopsies, the vessels showed a lack of staining with factor VIII in part of the vessel wall of a large number of sinusoids. Whether this is a lack of endothelial lining or a lack of expression of factor VIII needs further evaluation. At this moment we tend to think the endothelial lining is actually missing. McDougall, et al. also observed this phenomenon and described it as a disruption of the endothelial cells (16). It is not clear whether the lack of vasoconstriction is a result of changes in the vessel itself or due to the decrease of nerves in the nasal mucosa, since the number of neurofilaments were extensively reduced in all leprosy groups compared to normal controls. More reduction occurred in the borderline/lepromatous group than in the tuberculoid group. Although neurofilaments were reduced in all groups, confirming the observation that a diminished sense of smell and taste occurred in leprosy patients throughout the leprosy spectrum (34), septal perforations were only found in the lepromatous leprosy patients and in two patients of the borderline group (BL). This might indicate that nose picking and sensitivity disorders are not the main reason for the occurrence of septal perforations but, more likely, the cause of mucosal destruction is by nonmechanical means.
The findings concerning the composition of the cellular infiltrate are puzzling, although some can be explained. In the skin lesions of leprosy patients the number of CD4+ cells is higher in tuberculoid and borderline than in lepromatous patients (18, 28, 32, 33) However, in the nose the number of CD4+ cells is found to be higher in the lepromatous mucosa. A possible explanation could be that these CD4+ cells have nothing to do with the actual leprosy infection and are not directed against M. leprae antigens but directed against the abundant superinfection encountered in the damaged mucosa. This superinfection can also be held responsible for the large number of CD 15+ cells (granulocytes) in the epithelium. The number of these cells is not increased in the lamina propria, reflecting the observation that none of the patients had erythema nodosum Ieprosum at the moment of the biopsy.
An increased number of CD68+ cells was found in the lamina propria of lepromatous patients. This fits with the observation in the skin that the dermis of lepromatous patients contains granuloma consisting mainly of macrophages (28).
The most striking observation is the decreased number of CD8+ cells in the mucosa of all leprosy patients independent of the classification. The number of these cells declines over the leprosy spectrum from tuberculoid to lepromatous; moreover it declines also with the duration of the disease. The findings are independent of treatment; an independence which was also noticed by McDougall, et al. (16). However, their observation period was only 6 weeks. In patients having a long history, however, the number of CD8+ cells increased again, perhaps reflecting a slight improvement in immunity which also can be seen in the skin in long-term, adequately treated lepromatous patients (37).
Normally the epithelium of the nasal mucosa shows a considerable number of CD8+ cells. These cells in the nasal mucosa are thought to be cytotoxic, being involved in the first line of defense (4). Their relative absence in the mucosa of leprosy patients may contribute to the observed increase in CD4+ cells and granulocytes which may have to take over some of the tasks of the cytotoxic CD8+ cells.
In the skin of lepromatous leprosy patients the CD8+ cell is the predominant T cell, considered to be a suppressor cell responsible for the nonresponsiveness of lepromatous leprosy patients to M. leprae antigens. In the skin in leprosy the CD4/CD8 ratio decreases over the spectrum from tuberculoid to lepromatous, quite contrary to the findings in the nose where the CD4/ CDS ratio increases from tuberculoid to lepromatous. Whether the CD8+ cells in the skin in leprosy are actually suppressor cells and those in the nose actually cytotoxic cells has not yet been properly established.
It is not clear as yet whether the reduced numbers of CD8+ cells are acquired during infection or whether persons with a low number of CD8+ cells in their nose might have a higher risk of acquiring leprosy. This speculation requires further study, especially since it has been shown that normal individuals readily encounter M. leprae and that antigenic determinants, when presented via the nasal mucosa to the CMI system, are able to induce tolerance (12). Moreover, it has been shown that the CD8+ cells are of crucial importance for the acquired immunity against some intracellular pathogens (10, 13).
Acknowledgment. This study was made possible by the financial and material support of the International Order of the Knights of St. Lazarus of Jerusalem. The International Lazarus Leprosy Society. The Q.M. Gastmann-Wichers-Stichting, Glaxo Wellcome and Storz. Our thanks goes to the patients and staff of the Institute) Lauro de Souza Lima in Bauru and Santa Cruz in Rio de Janeiro. Brazil.
REFERENCES
1. CHACKO, C. K., BHANU, T, VICTOR, V, ALEXANDER, R., TAYLOR, P. M. and JOB, C. K. The significance of changes in the nasal mucosa in indeterminate, tuberculoid and borderline leprosy. Lepr. India 51 (1979) 8-22.
2. DAS, P. and NAAFS, B. Lepra: aetiologie. pathogenese en epidemiologic. In: Import Dermatologie. Faber. W. and Naafs. B., eds. Nieuwegein: Glaxo. 1995. pp. 31-36.
3. DE. VRIES. R. R. P., LAI A FAT. R.. NIJENHUIS. L. and VAN ROOD. J. HLA-linked genetic control of host response to Mycobacterium leprae. Lancet 2 (1976) 1328-1330.
4. FOKKKNS. W. J.. HOLM, A. F.. RIJNTJES. E.. MULDER. P. G. H. and VROOM, T. M. Characterization and quantification of cellular infiltrates in nasal mucosa of patients with grass pollen allergy, non-allergic patients with nasal polyps and controls. Int. Arch. Allergy Appl. Immunol. 93 (1990) 66-72.
5. FOKKENS, W. J., VKOOM. T. M., GERRITSMA. V. and RIJNTJES, E. A biopsy method to obtain high quality specimens of nasal mucosa. Rhinology 26 (1988) 293-295.
6. HANSEN, G. Undersogelser angaenda spedalskhedens arsager. Norsk Magazin for Laegevidenskaben 4 (1874) 1-88.
7. HUANG, C. L. The transmission of leprosy in man. Int. J. Lepr. 48 (1980) 309-318.
8. JOB, A. and CHACKO, C. J. G. Reactional states in the nasal mucosa: a clinical and histopathologic study. Int. J. Lepr. 56 (1988) 523-526.
9. JOB, C. K., KARAT, A. B. and KARAT, S. The his to-pathological appearance of leprous rhinitis and pathogenesis of septal perforation in leprosy. J. Laryngol. Otol. 80 (1966) 718-732.
10. KAUFMANN, S. H. E. Role of T-cell subsets in bacterial infections. Curr. Opin. Immunol. 3 (1991) 465-470.
11. KLATSER, P. R., VAN BEERS, S., MADJID, B., DAY, R. and DE WIT, M. Y. L. Detection of Mycobacterium leprae nasal carriers in populations for which leprosy is endemic. J. Clin. Microbiol. 31 (1993) 2947-2951.
12. KUPER, C. F., KOORNSTRA, P. J., HAMELEERS, D. M., BIEWENGA, J., SPIT, B. J., DUIJVESTIN, A. M., VAN BREDA-VRIESMAN, P. J. AND SMINIA, T. The role of nasopharyngeal lymphoid tissue. Immunol. Today 13 (1992) 219-224.
13. LADEL, C. H., DAUGELAT, S. and KAUFMANN, S. H. E. Immune response to Mycobacterium bovis bacille Calmette-Guerin infection in major histocompatibility complex class I- and Il-delicient knock-out mice: contribution of CD4 and CD8 T cells to acquired resistance. Eur. J. Immunol. 25 (1995)377-384.
14. LEIKER, D. On the mode of transmission of Mycobacterium leprae. Lepr. Rev. 48 (1977) 9-16.
15. LUNA, L. Manual of Histologic Staining Methods. New York: McGraw Health Book Co., 1968, pp. 230-232.
16. MCDOUGALL, A. C, REES . R. J., WEDDELL, A. G. and KANAN. M. W. The histopathology of lepromatous leprosy in the nose. J. Pathol. 115 (1975) 215-226.
17. MODLIN, R. L. Thl-Th2 paradigm: insights from leprosy. J. Inv. Dermatol. 102 (1994) 828-832.
18. MODLIN, R. L., HOFMAN, F. M., TAYLOR, C. R. and REA, T. H. In situ characterization of T lymphocyte subsets in leprosy granulomas. (Letter) Int. J. Lepr. 50 (1982) 361-362.
19. MODLIN, R. L., HOFMAN, F. M., TAYLOR, C. R. and REA, T. H. T lymphocyte subsets in the skin lesions of patients with leprosy. J. Am. Acad. Dermatol. 8 (1983) 182-189.
20. MODLIN, R. L., LEWIS, J., UYEMURA, K. and TIGELAAR, R. R. T lymphocytes bearing gamma-delta antigen receptors in skin. Chem. Immunol. 53 (1992) 61-74.
21. MYRVANG, B., GODAL, T, RIDLEY, D., FROLAND, S. and SONG, Y. Immune responsiveness to Mycobacterium leprae and other mycobacterial antigens throughout the clinical and histopathological spectrum of leprosy. Clin. Exp. Immunol. 14 (1973) 541-553.
22. NAAFS, B. Eziopatogenesi. In: Manuale di Leprologia. Nunzi, E. and Leiker D., eds. Bologna: OSCI, 1990.
23. NAAFS, B. and ALEMAYEEHU, A. Comparison of the modes of spread and the age at onset of leprosy and tuberculosis. Amsterdam: University of Amsterdam, 1980.
24. OTTENHOFF, T. H. M., GONZALEZ, N. M., DE VRIES, R. R. P., CONVIT, J. and VAN ROOD, J. J. Association of HLA specificity LB-E12 (MB1, DC1, MT1) with lepromatous leprosy in a Venezuelan populations. Tissue Antigens 24 (1984) 25-29.
25. PALLEN, M. J. and MCDERMOTT, R. D. How might Mycobacterium leprae enter the body? Lepr. Rev. 57 (1986) 289-297.
26. PEDI.EY, J. C. The nasal mucus in leprosy. Lepr. Rev. 44 (1973) 33-35.
27. PEDLEY, J. and GEATER, J. Does droplet infection play a role in the transmission of leprosy. Lepr. Rev. 47 (1976) 97-102.
28. REA, T. H. and MODLIN, R. L. Immunopathology of leprosy skin lesions. Semin. Dermatol. 10 (1991) 188-193.
29. REES. R. J. W. The impact of experimental human leprosy in the mouse on leprosy research. Int. J. Lepr. 39 (1971) 201-215.
30. REES, R. J. W. and MEADE, T. Comparison on the modes of spread and the incidence of tuberculosis and leprosy. Lancet 1 (1974) 47-48.
31. REES, R. J. W. and MEADE, T. Airborne infection with M. leprae in mice. J. Med. Microbiol. 10 (1977) 63-68.
32. SALGAME, P., YAMAMURA, M., BLOOM, B. R. and MODLIN, R. L. Evidence for functional subsets of CD4+ and CD8+ cells in human disease: lymphokine patterns in leprosy. Chem. Immunol. 54 (1992) 44-59.
33. SEHGAL, V. N.. GUPTA, R. P., KARMAKAR, S., LOGANI, K. B. and JAIN, S. In situ characterization of lymphocytic immunophenotypes and interleukin-2 receptors in cutaneous tuberculosis and leprosy-a comparative evaluation. Clin. Exp. Dermatol. 19 (1994) 312-316.
34. STEGINK, A., ZOETEMAN, J., CHAU, E., FOKKENS, W. and NAAFS, B. Taste, smell and mucosal nasal sensitivity in leprosy (submitted for publication).
35. STONER, G., TOUW, J., BELEHU, A. and NAAFS, B. In-vitro lymphoproliferative response to Mycobacterium leprae of HLA-D-identical sibling of lepromatous leprosy patients. Lancet 2 (1978) 994-996.
36. TAKAHASHI, D., ANDRADE, H. F, JR., WAKAMATSU, A., MANINI, M. and DE BRITTO, T. Treated indeterminate leprosy: a search for predictive histopathological and immunohistochemical parameters in skin biopsies taken from patients at admission and at clinical discharge. Acta Leprol. 8 (1992) 95-102.
37. TURK, J. L. and WATERS, M. F. W. Immunological basis for depression of cellular immunity and the delayed allergic response in patients with lepromatous leprosy. Lancet 2 (1968) 436-438
38. VAN EDEN, W., DE VRIES, R. R. P., MEHRA, N. K., VAIDYA, M. C, D'AMARO, J. and VAN ROOD, J. J. HLA segregation of tuberculoid leprosy: confirmation of the DR2 marker. J. Infect. Dis. 141 (1980) 693-39. 702.
39. VAN VOORHIS, W. C., KAPLAN G., SARNO, E.N., HORWITZ, M.A., STEINMAN, R.M., LEVIS, W.R., NOGUEIRA, N., HAIR, L. S., GATTAS, C. R., ARRICK, B. A. and COHN, Z. The cutaneous infiltrates of leprosy: cellular characteristics and the predominant T cell phenotypes. N. Engl. J. Med. 307 (1982) 1593-1597.
40. VIDAL, S. M., PINNER, E., LEPAGE, P., GAUTHIER, S. and GROS, P. Natural resistance to intracellular infections: (Nrampl D169) mouse strains. J. Immunol. 157 (1996) 3559-3568.
1. M.D., Ph.D.; Department of Otorhinolaryngology, Erasmus University Medical Center, Dr. Molewaterplein 40, 3015 GD Rotterdam, The Netherlands.
2. B.Sc., Department of Otorhinolaryngology, Erasmus University Medical Center, Dr. Molewaterplein 40, 3015 GD Rotterdam, The Netherlands.
3. M.D., Ph.D., Department of Otorhinolaryngology, Academie Medical Center, Amsterdam, The Netherlands.
4. M.D., Ph.D., Instituto Lauro de SouzaLima, Bauru, S.P., Brazil.
5. M.D.,Ph.D., Ministry of 1-1ealth, Brasilia, Brazil.
6. M.D., Department of Otorhinolaryngology, Waterland Hospital, Purmerend, The Netherlands.
7. M.D., Ph.D., Department of Dermatology, Leiden University Medical Center, The Netherlands.
Reprint requests to Dr. Fokkens at the above address or fax 31-10-463-3102.
Received for publication on 17 March 1998.
Accepted for publication in revised form on 24 August 1998.