- Volume 66 , Number 3
- Page: 340–7
Contribution of type 1 reactions to sensory and motor function loss in borderline leprosy patients and the efficacy of treatment with prednisone
ABSTRACT
The changes in nerve function tests in 297 new leprosy patients over an average period of 30 months were measured. The impact of type 1 reactions (T1R) on sensory and voluntary muscle function was measured by standard tests. Sensory function was improved in patients with single episodes of cutaneous T1R, but not improved in patients with neural T1R or with multiple episodes of either kind of T1R. Patients over 40 years of age improved less than younger patients, and patients admitted for treatment of T1R improved more than those treated as outpatients. These data point to a need to find better regimens for the treatment of nerve damage in T1R.RÉSUMÉ
Les variations observées à partir des tests neurologiques fonctionnels de nerfs périphériques ont été mesurées chez 297 nouveaux patients atteints de lèpre sur une période moyenne de 30 mois. L'importance des réactions de type 1 (RT1) sur la fonction sensorielle et musculaire volontaire a été évaluée en utilisant les résultats obtenus à partir de tests standards. La fonction sensorielle s'est améliorée chez les patients présentant des épisodes uniques de RT1 cutanées. Elle ne s'est pas améliorée chez les patients souffrant de RT1 nerveuses ou d'épisodes multiples de chaque type de RT1. Les patients de plus de 40 ans ont présenté une amélioration moins nette que les patients plus jeunes, et les patients hospitalisés pendant leur traitement contre la RT1 ont joui d'une amélioration plus importante que ceux qui ont été traités à la maison. Ces données suggèrent qu'il y a un besoin pressant de trouver de meilleures modalités pour le traitement des atteintes nerveuses associées aux RT1.RESUMEN
So midieron los cambios en las pruebas de función nerviosa en 297 pacientes con lepra, durante un periodo de 30 meses. El impacto de las reacciones tipo 1 (RT1) sobre las funciones sensorial y de los músculos volutarios se midió utilizando pruebas estándar. La función sensorial mejoró en los pacientes con episodios únicos de RT1 cutáneas pero no mejoró en los pacientes con RT1 neural es o con episodios múltiples de RT1 de cualquier tipo. Los pacientes de más de 40 años de edad mejoraron menos bien que los pacientes más jóvenes y los pacientes admitidos para tratamiento de RT1 mejoraron más que los tratados como pacientes externos. listos datos señalan la necesidad de encontrar mejores esquemas para el tratamiento del daño a nervios en las RT1.The prevention and treatment of reactions and nerve damage has recently been highlighted as the top research priority in leprosy (1). Much of the nerve damage in leprosy is thought to take place during reactions (4). There have, however, been very few, large, long-term assessments of the impact of type 1 reactions (T1R) on nerve function nor on the effectiveness of current prednisone treatments for T1R in terms of nerve function recovery.
The aim of this paper was to quantify the changes in sensory and motor functions in a cohort of new borderline leprosy patients with and without episodes of T1R in order to assess the contribution that these episodes make to nerve function, impairment and the efficacy of a semi-standardized prednisone treatment regimen.
MATERIALS AND METHODS
Patients
This retrospective study consisted of 360 borderline leprosy (BT, BB, BL) patients, diagnosed on clinical and bacteriological grounds according to the Ridley-Jopling classification (9), who presented at Anandaban Leprosy Hospital between 1989 and 1996. These patients had had no previous antileprosy treatment, were regular patients at Anandaban Hospital, and had had sensory and voluntary muscle testing performed at diagnosis and on a second occasion at least 6 months later.
Type 1 reactions were diagnosed as neural when there was marked tenderness, weakness, paralysis or anesthesia of less than 3 months' duration. In practice, patients with nerve function loss but having no nerve pain or tenderness or other signs of reaction were not included. Cutaneous reactions were characterized by inflamed plaques, erythematous lesions with raised edges (with or without scaling), ulceration, edema in hands or feet, an inflamed facial patch, or the appearance of new lesions during multiple drug therapy (MDT). Patients with erythema nodosum leprosum (ENL) nodules were not included.
Sensory and voluntary muscle testing
Sensory testing (ST) was performed at 14 points on the palms of each hand and the sole of each foot by the ball-point pen test (12). The sensation of the cornea was tested with sterile cotton wool. For this study, definite sensation was scored as 1 and doubtful or insensitive as 0. Total scores for the hands and feet of 28 and for the eyes of 2 were recorded and combined in a cumulative sensation score with a maximum of 58.
Voluntary muscle testing (VMT) was performed at the same time as sensory testing by standard methods (3). This VMT gave a score of 15 for each hand based on little finger abduction, thumb abduction and wrist extension. A score of 10 for each foot was based on foot dorsiflexion and eversión. The strength of eye closure gave a maximum score of 5 for each eye. Scores were combined to give a cumulative VMT score with a maximum of 60.
ST and VMT scores at presentation and at the most recent testing (at least 6 months after initial testing) were compared using a grading previously described (11). Scores on the second testing more than 1 point higher than those at diagnosis were regarded as improvement; those patients with a score less than 1 point lower than their initial score were regarded as worsening.
Disability classification was performed on all patients at their initial consultation using the World Health Organization (WHO) 1977 disability index (Dl) (13) and in 278 patients on a second occasion at least 6 months later.
Statistics
The significance of changes in ST, VMT and DI in individual patients was tested by the paired Wilcoxon Z test. Differences in proportions were compared by means of the chi-squared test. Differences in the ST and VMT scores between patient groups were tested by the Mann-Whitney U test.
RESULTS
Of the 360 patients enrolled in this study, 63 were excluded from further analysis. These 63 included 24 patients with T1R who had no details of antiinflammatory treatment and 39 patients with sensory or motor function loss of less than 6 months' duration, without symptoms of T1R, who were being treated with prednisone.
The final group consisted of 297 patients, 205 of whom were male and 92 were female with ages ranging from 7 to 78 years; 143 were classified as borderline tuberculoid (BT), 27 as true borderline (BB) and 127 as borderline lepromatous (BL).
Of the 297 patients 157 (53%) experienced a T1R reaction during the follow-up period (average follow up 30.7 months; range 6 to 74 months). Patients who experienced a T1R reaction were followed for a longer period (33.4 months) than those patients without T1R (27.6 months, p <0.05). The T1R reaction was cutaneous in 68 cases, neural in 37 and mixed (both cutaneous and neural) in 52 cases. There were no significant differences in the prevalence of T1R between male (107/205, 52%) and female (50/92, 54%) patients or between younger (<40 years) or older (>40 years) patients (93/176, 53% versus 64/121, 53%). There was a significantly lower proportion of T1R among BT patients (51/143, 36%) than either BB [17/27 (63%), p <0.05] or BL [89/127 (70%), p <0.001] patients.
The majority of patients (111, 71%) had a single episode of T1R; 29 patients (18%) had two episodes, 11 (7%) had three episodes, 5 (3%) had four episodes and 1 patient had five episodes. These were distinct episodes following at various times after completion of treatment for Tl R. There was no significantly greater recurrence of T1R in patients with neural or mixed T1R (25/89), compared with patients with cutaneous T1R (21/68).
The length of untreated T1R was recorded in 67 cases. While 73% of all T1R cases were treated within 2 months of the symptoms appearing, 12/27 (44%) of the patients with neural T1R compared with 6/30 (30%, p = NS) patients with cutaneous T1R had symptoms for 3 months or longer before reporting for and receiving antiinflammatory treatment.
Ten patients had relapsing T1R during which symptoms increased while under treatment and the dose and duration of treatment had to be increased (mean treatment length 21.3 weeks); five patients had cutaneous T1R and five had neural or mixed TlR(p = NS).
Treatment
All T1R patients were treated with prednisone and 86% of them were treated with a maximum dose of 40 mg daily, reduced in steps over a period of 4 to 74 weeks (average 15.8 weeks). Generally, two schedules were followed: a) in the earlier part of the study, patients received 40 mg reduced by 5 mg weekly over a period of 8 weeks, and b) later most patients received 40 mg for 2 weeks followed by 30, 20, 15, 10 and 5 mg for a total of 12 weeks. However, the length of treatment varied with individual patients. Ten patients were treated with a maximum 60-80 mg daily and 12 were treated with a 20-30 mg maximum for an average of 12 weeks. Patients with skin T1R symptoms were treated for an average of 17.5 weeks; those with mixed cutaneous and neural T1R for an average of 16.5 weeks. Patients with neural T1R were treated for significantly shorter periods (average 11.5 weeks, p <0.01). The mean maximum dose for patients with neural T1R (41.62 mg) was not significantly higher than that given to patients with skin T1R (40.15 mg).
Of the 157 patients with T1R, 99 were admitted to our hospital for treatment for periods of between 1 and 30 weeks (average stay 7 weeks); the remainder were treated as outpatients.
Sensory and motor function
The changes in ST and VMT scores in patients in various clinical groups are shown in Tables 1 and 2. No significant differences were found between BT, BB or BL patients. The differences between left and right hands, feet or eyes were found to be not significant (data not shown).
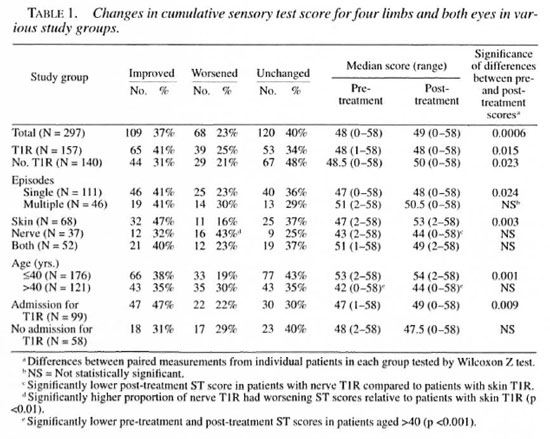
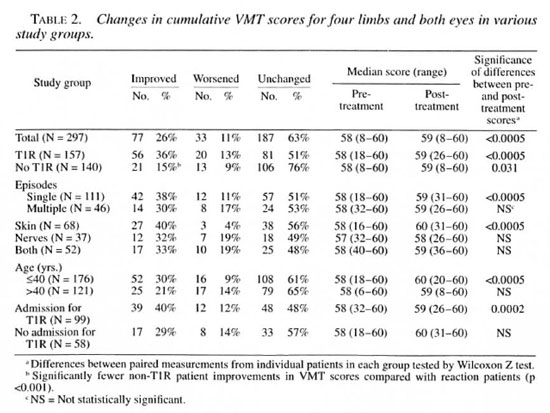
Of the 297 patients, less than half (117, 39%) improved in either ST or VMT scores; 51 patients (17%) improved in both ST and VMT scores, while 15 (5%) improved in VMT scores only, and 51 (17%) improved in ST scores only. Sensory and VMT changes in individual patients were significantly correlated (p <0.001).
Of patients with completely anesthetic hands, 8/18 (44%) recovered some sensation; 16/59 (27%) of patients with anesthetic feet and 3/20 with corneal anesthesia also recovered some sensory function. Of the 7 patients with paralyzed hands or feet or lagophthalmus, only 1 patient with a paralyzed hand and 1 patient with lagophthalmus recovered some muscle function; 4 patients with paralyzed feet did not recover any muscle function.
Sensory scores. The sensory scores in hands and feet were significantly improved in the total group over the study period (Table 3 and data not shown). There was no improvement in corneal sensation (data not shown) but an over-all improvement in the cumulative sensory score (Table 1).
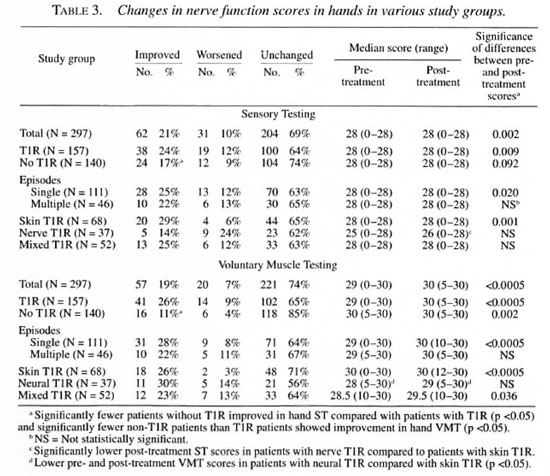
When patient subgroups were considered, sensation improved overall in patients with and without T1R (Table 1). However, patients with multiple T1R episodes or with neural T1R (with or without skin involvement) did not show any significant improvement. The improvements seen were largely contributed to by improvements in hand ST scores; sensory scores in feet and eyes did not reach significance for any subgroup (Table 3 and data not shown). The magnitude of the difference in post-treatment sensory scores between different subgroups was further tested by the Mann-Whitney U test. Differences reached statistical significance between skin and neural T1R patients for hand and cumulative sensory scores (Tables 1 and 3, p <0.05). There were significantly fewer patients showing improvement in sensory scores in the neural T1R group compared with the skin T1R group (Table 1), and fewer T1R patients improved their hand sensory scores compared with non-T1R patients (Table 3).
Voluntary muscle testing (VMT). Voluntary muscle test scores improved for the whole patient group for hands (Table 3) and in the cumulative total (Table 2). VMT scores did not improve in feet and eyes (data not shown).
The changes in VMT for patient subgroups were greatest in the non-T1R patients and in those T1R patients with single, cutaneous reactions. Patients with multiple or neural T1R did not improve their VMT scores in hands, feet or eyes. The magnitude of the difference in post-treatment VMT scores between different subgroups was further tested by the Mann-Whitney U test. There were significantly lower median VMT scores for hands in those with neural T1R compared with those with skin T1R (Table 3). However, the pre-treatment VMT scores were also significantly lower. The proportion of patients improving their VMT scores was lower in non-T1R patients compared with T1R patients in the cumulative VMT score (Table 2); this reflected significantly fewer patients who improved in hand and foot VMT scores (Table 3 and data not shown).
Other factors influencing nerve function, van Brakel suggested that men with nerve function loss improved their VMT scores significantly better than women (11). An analysis of all sensory and motor function scores by sex showed no significant difference between men and women. However, a significant difference was shown between patients older than 40 years and those aged 40 years or younger. While the prevalence of T1R was the same between the age groups, older patients failed to improve either their cumulative ST or VMT scores. The median sensory scores were significantly lower, both at diagnosis and after treatment, in the older group (Table 1). When the outcomes for those with reaction in the two age groups were compared, only younger patients showed improvements in cumulative ST and VMT scores (data not shown).
We also investigated the effect of admission to our hospital on the cumulative ST and VMT scores of patients with T1R. While the two groups were similar in composition (that is, there were no significant differences in the distribution of neural cases between admitted and nonadmitted cases), the outcomes for nonadmitted T1R cases were significantly poorer. Thus, ST and VMT scores for admitted patients were significantly improved, while the nonadmitted patients' ST and VMT scores were unchanged (Tables 1 and 2).
The effect of the length of steroid therapy on nerve function outcomes was also investigated. However, since the mean length of therapy for neural T1R was significantly shorter than for cutaneous T1R, a valid comparison between shorter and longer treatment times could not be made.
Disability grading
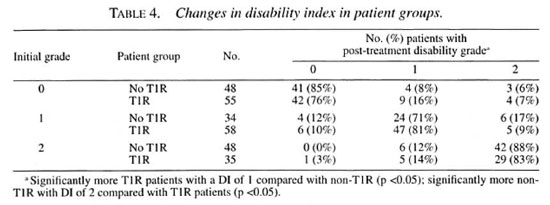
Table 4 shows the changes in disability grading for patients with and without T1R over the study period. While 13/55 (23%) of the T1R patients without initial disability developed some disability during the study period, only 7/48 (14.5%) nonreactional patients did so (p = ns). However, equal proportions of T1R patients (12/93, 13%) compared with nonreactional patients (10/82, 12%) with disability at presentation improved their disability grading.
DISCUSSION
This study has assessed the outcomes for patients with type 1 reactions (T1R) treated with a semi-standardized course of a maximum dosage of 40 mg prednisone compared with a nonreactional historical control group. We have used a conservative measurement of sensory nerve function, namely, touch thresholds as determined by the ball-point pen test. This test does not measure milder nerve impairment, and, together with a conservative categorization of change (i.e., a change of more than 1 in any measure), tends to emphasize the validity of the outcomes seen. The internal consistency of measurements by trained physiotherapy technicians is high (11), allowing us to compare sensory and motor function tests conducted (often by the same technicians) over an average follow-up period of 30 months. Significant improvements in ST and VMT scores were evident, particularly in the hands. Improvements in foot muscle strength, corneal sensation and lid closure strength were not significant.
The improvements in the T1R patients' cumulative ST and VMT scores and sensory scores in hands were greater than in nonreactional patients. However, these improvements were limited to T1R patients who had experienced a single reactional episode and whose reaction was limited to the skin. Patients with multiple episodes of reaction and reactions involving nerves with or without skin symptoms did not improve significantly in either ST or VMT scores. Patients developing T1R reactions in nerves tend to present later after the start of MDT (10) and, in this study, to have had symptoms of T1R for longer than did those with cutaneous symptoms. These patients were also treated for significantly shorter periods than were those patients with cutaneous T1R, possibly because of a more rapid diminution in symptoms. However, these results would seem to indicate that there is continuing nerve damage after the disappearance of symptoms. Longer treatment and perhaps higher doses of steroids are indicated for the successful treatment of neural T1R (7).
Patients who were treated as inpatients during their reaction improved significantly more than those treated as outpatients. Hospital inpatients benefitted from additional health education, splinting and bedrest during reactions, and these factors had an obvious impact on recovery of nerve function. An unpublished survey of the completion rates for outpatients taking steroids at this hospital suggests that between 20%-30% of outpatients fail to collect their full course of steroids. Patients older than 40 years of age had no improved nerve function when compared with their younger counterparts. This is, in part, due to a lower initial ST score in older patients; however, the recovery of nerve function was also insignificant. Among older patients with T1R, in contrast to younger patients, there was also no significant improvement in nerve function.
It is significant that there is a decline in sensation (21%) and muscle strength (13%) in nonreactional patients. This indicates a chronic nerve function loss often called silent neuritis which is important to diagnose and treat in order to further reduce nerve impairment due to leprosy, van Brakel (11) has reported a prevalence of 7% for silent neuropathy with 71 % of episodes occurring in the first year of MDT; hence, some of the patients in this present study were undoubtedly affected. These patients have been shown to respond well to steroid treatment, with recovery of nerve function.
Differences in the definition of T1R limit the ability to compare our results with those of other workers. We have used consensus criteria developed for a current randomized control trial of fixed-duration low- versus high-dose prednisone treatment trial (Waters, personal communication). This categorization of T1R as cutaneous, neural or mixed has revealed important differences in the epidemiology and risk factors associated with these different forms of T1R (10), and now reveals that current treatment regimens are inadequate for those patients with neural T1R.
Modern long-course treatment of T1R arose from a study comparing two cohorts of patients in Ethiopia (8). Small-scale studies (5) gave support to the idea that nerve function could be recovered with steroid treatment of T1R. A large study of the application to the field showed a recovery of some function in 74% of reactional patients. However, these patients were selected by a positive response to steroids, with poor responses being hospitalized and removed from the study. Hence, the proportion of patients with nerve function improvement were much greater than seen here (5).
A comparable retrospective study in India reported on nerve function in 44 T1R patients over a 6-year period (6). A bigger improvement in sensory and motor function was found in patients with cutaneous T1R (93%) than in patients with neural T1R (50%) after treatment with 10-30 mg prednisone. No significant differences were noted in the improvement in different nerves or in patients receiving higher steroid doses.
Our results would lend support to a recent review which recommended extended high-dose prednisone treatment of longer than 3 months' duration (7). However, the suggested use of courses in BT patients of 3-6 months, in BB patients of 6-9 months, and in BL patients of 18-24 months would not seem to be justified by our data. There were no differences between leprosy classes in response to the regimens in the present study, and no significant differences in the prevalence of different kinds of T1R (cutaneous, neural or mixed) between different leprosy classes (10). Extended prednisone treatment should be on the basis of the extent of involvement of nerves in TI R since patients with T1R in nerves are not improving nerve function under the current regimens.
The ongoing trial of high- and low-dose, fixed-duration prednisone treatment for T1R will provide information on the effect of dosages of prednisone on skin, nerve and mixed T1R. Better measurements of the effect of antiinflammatory drugs on nerve function along with better antireaction drugs may be required, particularly for neural T1R.
Acknowledgment. This work was supported by The Leprosy Mission International. The authors would like to thank the staff and patients of Anandaban Hospital for their cooperation in this study.
REFERENCES
1. ANON. Setting priorities. Int. J. Lepr. 64 Suppl. (1996) S91-S92.
2. BECX-BLEUMINK, M. and BERHE, D. Occurrence of reactions, their diagnosis and management in leprosy patients treated with multidrug therapy: experience in the leprosy control program of ALERT in Ethiopia. Int. J. Lepr. 60 (1992) 173-184.
3. BRANDSMA, W . Nerve function assessment in leprosy patients. Lepr. Rev. 52 (1981) 161-170.
4. JOB, C. K. Nerve damage in leprosy. Int. J. Lepr. 57 (1989) 532-539.
5. KIKAN. K. U.. STANLEY, J. N. A. and PEARSON, J. M. H. the outpatient treatment of nerve damage in patients with borderline leprosy using a semi-standardised steroid regimen. Lepr. Rev. 56 (1985) 127-134.
6. LOCKWOOD, D. N. J., VlNAYAKUMAR, S., STANLEY, J. N. A., MCADAM, K. P. W. J. and COLSTON, M. J. Clinical features and outcome of reversal (type 1) reactions in Hyderabad, India. Int. J. Lepr. 61 (1993) 8-15.
7. NAAFS, B. Treatment of reactions and nerve damage. Int. J. Lepr. 64 Suppl. (1996) S21-S28.
8. NAAFS, B., PEARSON, J. M. H. and WHEATE, H. W. Reversal reaction: the prevention of permanent nerve damage; comparison of short- and long-term steroid treatment. Int. J. Lepr. 47 (1979) 7-12.
9. RIDLEY, D. S. and JOPLING, W. H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34 (1966) 255-273.
10. ROCHE, P. W., LE: MASTER, J. W. and BUTLIN, C. R. Risk factors for type 1 reactions in leprosy. Int. J. Lepr. 65 (1997) 450-455.
11. VAN BRAKEL, W. H.. Ph.D. thesis. University of Utrecht, 1994.
12. WATSON, J. M. Essential action to minimise disability in leprosy patients. London: The Leprosy Mission. 1986.
13. WHO EXPERT COMMITTEE ON LEPROSY. Fifth Report. Geneva: World Health Organization. 1977. Tech. Rep. Ser. 607.
1. Ph.D.; Mycobacterial Research Laboratory, Anandaban Leprosy Hospital, P.O. Box 151, Kathmandu, Nepal.
2. M.D.; Mycobacterial Research Laboratory, Anandaban Leprosy Hospital, P.O. Box 151, Kathmandu, Nepal.
3. M.B.B.S., Mycobacterial Research Laboratory, Anandaban Leprosy Hospital, P.O. Box 151, Kathmandu, Nepal.
4. M.B.B.S., United Mission to Nepal, P.O. Box 126, Kathmandu, Nepal.
Present address for Dr. Theuvenet: Koningnmelaan 23,7315 BK Apeldoorn. The Netherlands.
Reprint requests to Dr. Roche at the above address or FAX 977-1-290-538; email roche@umn..mos.com.np
Received lor publication on 6 April 1998.
Accepted lor publication in revised form on 21 July 1998.