- Volume 66 , Number 3
- Page: 356–64
Assessment of anti-PGL-I as a prognostic marker of leprosy reaction
ABSTRACT
The anti-phenolic glycolipid-I (PGL-I) assay as currently applied for leprosy is conceived as an early marker of asymptomatic infection, early disease diagnosis and cure monitoring. Its use as a prognostic marker of reaction is still a matter of controversy. We conducted a case-control study to investigate whether IgM and IgG anti-PGL-I antibodies could discriminate patients at increased risk of developing reactions. Eligible cases were untreated leprosy patients at the onset of type 1 and type 2 reactions recruited f rom among 600 concurrent, newly detected, untreated leprosy patients attending an outpatient clinic in central Brazil. For the patients with reaction, approximately the same number of leprosy cases without reaction matched as to bacterial index (BI), age and gender were randomly selected. Individuals without clinical leprosy were evaluated as healthy controls. Sera f rom type 1 reaction (N = 43) and type 2 reaction (N = 26) patients were tested by an ELISA using PGL-I synthetic disaccharide-BSA antigen and 1:300 sera dilution (cut-off point >0.2 OD). Antibody profiles were evaluated by exploratory data analysis and reverse cumulative distribution curves. The IgG anti-PGL-I response did not have a defined pattern, being detected only at low levels. Our results indicate that leprosy patients, independently of their reactional status, produce high levels of IgM anti-PGL-I, demonstrating a strong correlation between the magnitude of antibody response and the BI. Patients with a higher BI were at least 3.4 times more prone to produce an antibody response compared to healthy controls.RÉSUMÉ
Un marqueur précoce d'infection asymptômatique. une aide au diagnostic précoce de la maladie et au suivi de guérison représentent les applications communes actuelles, en ce qui concerne la lèpre, du test in vitro évaluant les anticorps anti-glycolipides phénoliques de type I (PGL-I). Son utilisation comme marqueur prognostique des réactions est encore sujet à controverses. Nous avons, de ce fait, mené une étude de cas contrôlés, afin de savoir si les niveaux d'anticorps, de type IgM et IgG, anti-PGL-I pouvaient permettre de détecter les patients susceptibles de développer des réactions. Les cas sélectionnés étaient des patients non-traités souffrant de lèpre, en début de réactions de type 1 ou de type 2, sélectionnés à partir de 500 patients lépreux non-hospitalisés nouvellement détectés, encore non-traités, se présentant à la consultation d'une clinique localisée dans la partie centrale du Brésil. Il fut sélectionné au hasard un nombre approximativement identique de cas de lèpre san réaction à l'index bactérioscopique (Bl). à l'âge et au genre comparables aux patients avec réactions. Des individus sans lèpre clinique furent évalués comme contrôles sains. Les sérums provenant de patients souffrant de réactions de type 1 (N = 43) et de réactions de type 2 (N = 26) furent testés par un ELISA utilisant l'antigène BSA-PGL-I disaccharide synthétique et und dilution à 1:300 du sérum (point limite de positivité 20.2 OD). Les profiles d'anticorps furent analysés en utilisant une analyse de données de type exploratrice des courbes de distribution cumulatives inverses. La réponse d'IgG anti-PGL-I ne montrait pas de profil bien défini, n'étant détectée qu'à de faibles niveaux. Nos résultats indiquent que les patients atteints de lèpre, indépendeniment de leurs états réactionnels, produisent de hauts niveaux d'IgM anti-PGL-I, mettant en évidence une forte corrélation entre l'importance de la réponse en anticorps et le BL Les patients ayant un haut Bl avaient au moins 3,4 fois plus de chance de produire lien réponse en anticorps que les contrôles en bonne santé.RESUMEN
El ensayo de los anticuerpos anti-glicolípido fenólico-I (PGL-I) en la lepra se ha usado como un indicador temprano de infección asintomática, como una prueba de diagnóstico temprano y como un monitor de la evolución de la enfermedad. Sin embargo, su uso como prueba de pronóstico de la reacción leprosa es aun controversia!. Nosotros hicimos un estudio controlado para investigar si los niveles de anticuerpos anti PGL-I IgM e IgG podrían servir para detectar aquellos pacientes en riesgo de desarrollar reacciones. Los casos elegibles fueron pacientes no tratados con reacciones de los tipos I y 2, seleccionados de entre 600 casos nuevos atendidos en una clínica del centro de Brasil. También se estudió un número similar de casos de lepra sin reacción leprosa pero comparables en edad, sexo y grado de afección. Así mismo se evaluó un grupo control de individuos sanos. Los sueros de los pacientes con reacción de tipo I (n = 43) o con reacción de tipo 2 (n = 26), y los sueros de los controles sanos se probaron en un ensayo de ELISA usando como antígeno un disacárido sintético (análogo del PGL-I) acoplado a BSA, y una dilución 1:300 de los sueros (D.O. = 0.02 como valor de corte). Los perliles de anticuerpos fueron evaluados por análisis de extrapolación de datos y por las curvas reversas de distribución acumulativa. La respuesta en IgG no tuvo un patrón definido y fue de bajo nivel. Nuestros resultados indicaron que los pacientes con lepra, independientemente de su estado reacciona!, produjeron niveles elevados de IgM anti-PGL-I, y mostraron una fuerte correlación entre la magnitud de la respuesta humoral y el índice bacteriológico (BI). Los pacientes con BI elevados produjeron niveles de anticuerpos cuando menos 3.4 veces más altos que los controles sanos.Both the occurrence and prognosis of leprosy as a chronic mycobacterial disease are changing rapidly due to simultaneous better therapeutic schedules, earlier diagnosis, near complete case finding, higher compliance to shorter duration schedules, and the possible modification of risk prevalence factors in most endemic regions (23). Although there is a perspective of achieving the elimination goal in a short period, the acute inflammatory episodes of leprosy, type I and type 2 reactions, still remain a challenge for case diagnosis, case management, a major cause of disability during and after specific treatment and. consequently, a burden for the public health services (19, 33).
A generally accepted dogma in immunology is that the magnitude of humoral and cell-mediated immune (C'MI) responses tend to be inversely related and that the CMI responses are crucial for immune protection and pathology of the mycobacterial infections (9, 15). Thl and Th2 CD4+ lymphocytes dictate, through their cytokines, the macrophage activation and the different outcomes of the clinical spectrum. Tuberculoid leprosy patients exhibit localized lesions, low bacillary load and an expansion of Thl cells that lead to macrophage activation. To the contrary, the lepromatous pole presents disseminated disease, high bacillary load and activation of Th2 cells which induce high levels of antibodies (7, 34). The type I reaction is considered a delayed-type hypersensitivity (DTH) response to bacillary antigens and presents upgrading of the clinical and histological patterns toward the tuberculoid pole; the type 2 reaction suggests an immune complex deposition phenomenon (24). Although important advances have occurred at the molecular level in identifying T-cell phenotype, cytokines and the dynamic immune events during the reactions, these techniques remain too complex to be applied at population level.
Currently, the detection of the species-specific antibody to phenolic glycolipid-I (PGL-I) in leprosy (3) has been widely evaluated in community surveys, leprosy contacts, early diagnosis and monitoring therapy (10, 11, 17, 29, 32} Native PGL-I and its synthetic analogs containing antigenic carbohydrates (4) have been used in ELISA to detect antibodies, mainly of the IgM class (5, 6). Antibody production to glycolipids is considered to be T-cell independent, thus mainly of the IgM isotype, but PGL-I was also demonstrated to induce the IgG isotype (29). Results from previous PGL-I studies have demonstrated that the technique is sensitive for multibacillary leprosy but detects from 30% up to 60% of paucibacillary cases (17). In general, 5%-10% of healthy individuals from leprosy-endemic areas have anti-PGL-I antibodies (2, 5, 8). Anti-PGL-I IgM antibodies have been correlated to the total bacillary load of leprosy patients and have been reported to be adequate to monitor therapy since antibody titers decline with treatment (1, 21). It has been suggested that this assay could be a useful tool to predict type 1 reaction (27), but its use as a prognostic marker of reaction is still a matter of controversy (17).
We conducted a case-control study to investigate whether IgM and IgG anti-PGL-I antibodies detected at the time of diagnosis could discriminate patients at increased risk of developing the first episode of reaction.
MATERIALS AND METHODS
This study is part of a research project conducted in the city of Goiânia located in a state highly endemic for leprosy in central Brazil; details of the area were previously reported (20). In brief, the baseline study recruited 600 newly detected leprosy patients from the main outpatient leprosy clinic during 12 months (1992/1993). At the same time, 552 healthy individuals were selected from among general clinics from seven health centers geographically located in areas where leprosy patients came from. The diagnosis of leprosy was based on clinical examination and a slit-skin smear collected for bacterial index (BI). For all smear-negative patients, skin biopsies were also collected and read by one pathologist. Both histopathologic and bacilloscopic readings were performed by experts from the Federal University of Goiás independently of the Leprosy Control Program (LCP) routine.
Leprosy subtypes were categorized according to Ridley-Jopling criteria (26) based on clinical presentation by one dermatopathologist with experience with leprosy. The BI of each patient was calculated by counting the acid-fast bacilli in slit-skin smears taken from the earlobe and skin lesions. Type 1 reaction was defined as an acute episode of leprosy, identified by the clinical characteristics of the skin lesions, erythematous lesions with raised edges, inflammation of plaques, ulceration of lesions, edema of the hands or feet and/or symptoms of nerve involvement. For some patients a low-grade fever with or without malaise was also detected at the time of diagnosis. Type 2 reactions were identified by small, inflammatory, red, swollen and highly tender nodules which developed suddenly on the skin or in the subcutis, accompanied by fever and malaise. Other symptoms, such as acute neuritis, arthritis, myalgia, orchitis and peripheral edema, were also inclusion criteria. Considering that the selection of leprosy participants was from among newly diagnosed leprosy patients, this study describes episodes of type 1 or type 2 reactions that occurred prior to specific leprosy treatment. Healthy controls were clinically examined by dermatologists to rule out leprosy. Those with suspicious lesions, anesthesia, nerve thickening or a history of household contact with a known leprosy case were excluded.
Serum samples were collected by venipuncture for leprosy patients before multidrug (MDT) intake and stored at -20ºC at the laboratory of the Federal University of Goiás (UFG). The same procedure for data collection and storage was applied for the healthy control group. The data described here, including the standardized clinical and laboratory results, were coded and entered into computer files at the regional research center.
For this study, the eligible cases were untreated leprosy patients at the onset of type 1 reaction (N = 45) and type 2 reaction (N = 31). All leprosy patients were newly detected untreated cases. The reason for including only untreated patients was to avoid bias due to the evaluation of a heterogeneous group of patients regarding types of intervention, different lengths of compliance to treatment, or previous reaction episodes. For the patients with reaction, approximately the same number of leprosy cases without reaction matched for BI, age (within 3 years), and gender was selected at random by a computer program. A second control group composed of healthy individuals (2 controls : 1 case) was also chosen at random and matched by age (within 3 years) and gender to those leprosy patients with reaction. For this study sera were available from: a) 43 type I reaction patients; b) 26 type 2 reaction patients; c) 59 leprosy patients without reaction, and d) 127 healthy controls.
All of the leprosy patients were newly detected untreated cases. Patients diagnosed with a BI of >0 were defined as multibacillary and received 2 years of MDT; otherwise they were classified as paucibacillary and received 6 months of MDT. Corticosteroids or thalidomide were given to patients with reactions associated with the MDT regimens.
Serological tests. IgM and IgG antibodies to PGL-I were assayed by ELISA. PGL-I synthetic disaccharide coupled to bovine serum albumin (BSA; provided by Dr. M. J. Colston) was used at 0.25 µg/ml (96 µ-bottom wells; Costar Corp., Cambridge, Massachusetts, U.S.A.) and serum samples were used at a 1/300 dilution. Goat anti-human IgM-peroxidase (µ chain-specific; Sigma Chemical Co., St. Louis, Missouri, U.S.A.) and goat anti-human IgGperoxidase (Organon; Teknika Corp., West Chester, Pennsylvania, U.S.A.) were used at 1/3000 and 1/1000 dilutions, respectively. o-Phenylenediamine substrate-dye reagent was used at 0.4 mg/ml (Sigma). The optical density (OD) was read at 492 nm (ELISA reader; Behring Data Analysis Software EL 311). Results were expressed as the difference between the OD of the test serum from PGL-I antigen-coated plates minus the OD obtained from control BSA-coated plates which were processed exactly the same as the antigen-coated plates except for the presence of the antigen. Samples with absorbance >0.2 were considered positive based on the evaluation of healthy individuals from this endemic region (mean value plus 3 standard deviations) to calculate the cut-off point (unpublished data). Each serum sample was tested blindly in duplicate, and a positive control consisting of six serial twofold dilutions of a pool of highly positive sera and a negative serum were tested in each plate. The serological tests were performed without the knowledge of the participant's status (whether leprosy patient or healthy individual) or any other laboratory result. All samples were tested at once at the UFG Laboratory of Immunology.
Statistical analysis. Exploratory data analyses were performed to determine the antibody profile stratified by leprosy patients with and without reaction and healthy controls (13). Chi-squared tests or Fisher's exact tests were calculated to examine differences in the categorical characteristics; / test or variance analysis were used to compare the means of antibody results. A mean value and 95% confidence interval (95% CI) for an anti-PGL-I response (IgG) was calculated for each group in order to compare a summary measure of antibody levels among the participants. The percent of coefficient of variation (%CV), which expresses the standard deviation (S.D.) as a proportion of the mean, was calculated as CV = (S.D./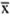 x 100 where
x 100 where 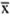 refers to the mean (12). The association of anti-PGL-I positivity and reaction was calculated as an odds ratio (OR) and 95% CI, crude and weighted OR, taking healthy individuals as the control group. Reverse cumulative distribution curves were plotted for IgM anti-PGL-I antibodies stratified by the participant's status (25). The sample size was considered sufficient to detect a 20%-25% difference in positive results comparing the type 1 or type 2 reactions with twice as many controls, at 5% significance and 80% power. Epi6 and SPSS were used for all analyses.
refers to the mean (12). The association of anti-PGL-I positivity and reaction was calculated as an odds ratio (OR) and 95% CI, crude and weighted OR, taking healthy individuals as the control group. Reverse cumulative distribution curves were plotted for IgM anti-PGL-I antibodies stratified by the participant's status (25). The sample size was considered sufficient to detect a 20%-25% difference in positive results comparing the type 1 or type 2 reactions with twice as many controls, at 5% significance and 80% power. Epi6 and SPSS were used for all analyses.
RESULTS
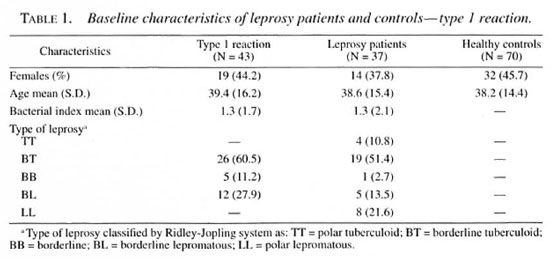
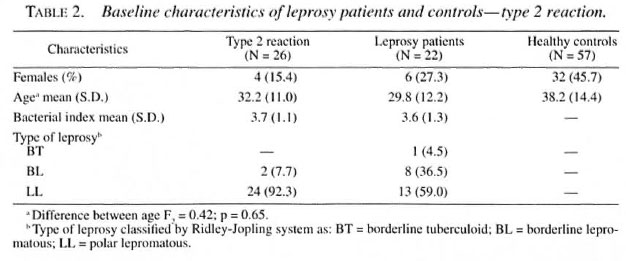
Among 600 newly detected leprosy patients 45 (7.5%) had developed a type 1 reaction and 31 (5.2%) a type 2 reaction at the time of leprosy diagnosis. Serological results were available for 90% of the patients with reactions, and exclusions were mostly due to refusal for blood collection. Baseline data regarding the leprosy patients, type 1 and type 2 reactions, age, sex and BI are presented in Tables 1 and 2. Reversal reactions were associated with borderline patients classified as borderline tuberculoid (BT; 60.5%), borderline lepromatous (BL; 27.9%) and mid-borderline (BB; 11.2%). Erythema nodosum leprosum (ENL) was mainly diagnosed in lepromatous (LL; 92.3%) patients. Healthy controls were similar in age and sex distribution to leprosy patients.
The box-plot diagrams in Figures 1 and 2 present the distribution of the OD readings for IgM anti-PGL-I for type 1 and type 2 patients and controls. The results indicate that healthy controls have lower IgM titers compared to leprosy patients independent of the reaction status. In fact the pattern of OD distribution is similar for leprosy patients with or without reactions but differs from the healthy controls distribution. OD values >0.67 for the healthy controls were considered outliers of the data distribution; whereas the median value for type I leprosy reaction was 0.75 (Fig. 1).
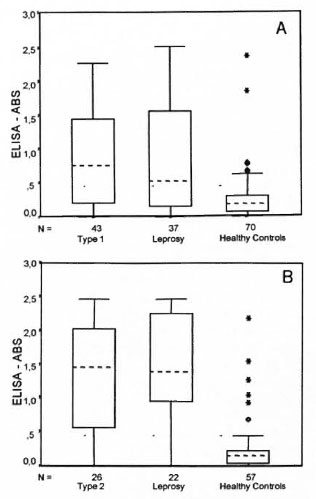
Fig. 1. Box-plot distribution for IgM antibodies anti-PGL-I. A = type 1 reaction, B = type 2 reaction.
Considering IgM OD results, the cumulative distribution curve for healthy controls showed low levels of antibody response; whereas the serological patterns for type 1 reaction and leprosy patients without reaction had similar profiles shifted to the right, indicating an increase in the proportion of leprosy patients with higher antibody levels (Fig. 2A). In Figure 2B a more pronounced shift to the right can be observed for leprosy patients, with similar profiles in leprosy with and without type 2 reaction, strikingly different from the curve distribution of the healthy participants. These results indicate that leprosy patients, independently of their reactional status, produce high levels of IgM anti-PGL-I antibodies. The advantage of plotting serum levels by the reverse cumulative distribution method is that there is no need for a prior assumption in order to report that the antibody distribution and visual comparison is straightforward (25).
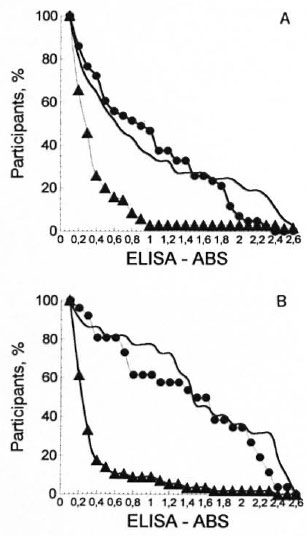
Fig. 2. Reverse cumulative distribution curves of IgM antibodies PGL-I among participants. A = type 1 reaction, B = type 2 reaction;  = leprosy patients.
= leprosy patients.  = controls.
= controls.
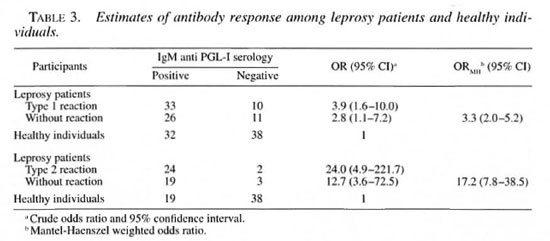
Using a cut-off point of 0.2 OD for PGL-I IgM antibody response, 76.7% of the patients during a type 1 reaction episode were seropositive compared to 70.3% of the leprosy patients without reaction (χ2= 0.43, p = 0.51). Compared with healthy controls, leprosy patients with and without reaction had an OR of 3.9 (95% CI 1.6-10.0) and 2.8 (95% CI 1.1-7.2), respectively, indicating statistically higher levels of antibody response among leprosy patients. There was a statistically significant difference in IgM seropositivity when comparing patients during a reversal reaction episode and healthy controls (%:= 10.5, p <0.001). Only two (7.7%) ENL and three (13.6%) of leprosy patients were seronegative compared to 66.7% of the healthy controls (% 2 = 42.0, p < 0.0, DF = 2) (Table 3).
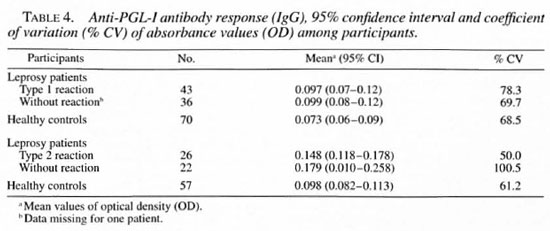
Serological results for the IgG anti-PGL-I response according to the participants are summarized in Table 4. Results of the IgG anti-PGL-I response were similar among reversal reaction patients, leprosy patients and the healthy controls with overlapping 95% CI. The percent of coefficient variation of antibodies (IgG) was above 50%, indi cating a high dispersion of OD measurements for all groups.
DISCUSSION
Our results indicate that newly diagnosed leprosy patients who develop the first episode of type 1 or type 2 reaction at the time of diagnosis elicit basically the same level of IgM anti-PGL-I antibody response as did the leprosy patients without reactions when matched for age, sex and BI. When serum levels were plotted by the reverse cumulative distribution method, the concentration of anti-PGL-I among leprosy patients, independent of the reaction status, was similar regarding the shapes and positions with intersect points that only reflect sampling variation. In contrast, healthy controls showed low median serological values and a completely different profile in comparison to leprosy patients according to the serum data depicted by box-plot and curve distributions.
It is generally accepted that there is a correlation between the BI and antibody titers, and that IgM anti-PGL-I could be applied as a surrogate serum marker of the patient's bacterial load, presenting high sensitivity and specificity when applied for multibacillary leprosy patients' evaluation (17). For this reason, the seronegative conversion of specific antibodies was considered useful for monitoring the clearance of the bacterial load, thus measuring the effect of MDT (1, 21). Our results support this evidence of a strong correlation between the OD and the BI. In fact, patients with a higher BI were at least 3.4 times more prone to mount an antibody response compared to the healthy controls.
In a previous follow-up study, a combination of higher concentration of IgM anti-PGL-I and lepromin positivity was suggested as a predictor of reversal reactions and was considered to be a useful tool for detecting leprosy patients at increased risk of developing type 1 reactions (28). One possible explanation of the discrepancy between our results and the results of the previous study is the study design. In the former cohort, the BI was considered a confounder variable and adjusted in the analysis stage. In our study, the basic assumption was that the bacillary load determines IgM antibody levels and, therefore, should be controlled at the design stage, being a possible effect modifier in the association of antibody and reaction. Our findings clearly show the similarity of serum patterns among leprosy patients, regardless of their reaction status when matched by the BI.
More recently, anti-PGL-I seropositivity and other risk factors were found to be associated with cutaneous type 1 reaction and neural type 1 reaction episodes that occurred prior to, during and after treatment in a retrospective cohort of leprosy patients (27). Different study designs and selection of patients may have accounted for the variation between these previous findings and ours. Considering that the correlation between the BI and IgM anti-PGL-I has been shown in our study to be in agreement with others (17, 27, 28), it is expected that matching cases and leprosy controls for BI would eliminate the effect of PGL-I seropositivity. Although sequential serum measurements would be helpful to understand the dynamics of antibody production during the course of leprosy, our findings show that the assessment of IgM anti-PGL-I was not useful in predicting patients during the first episode of reversal reaction or erythema no dosum leprosum prior to treatment.
Our results permit us to draw a clear pattern of the antibody profile directly associated with the BI; however, a few healthy participants presented high serum levels and some leprosy patients had low values. It is important to note that this investigation was conducted in a highly endemic region, and the probability of exposure to Mycobacterium leprae was also high and, therefore, subclinical infection may not be ruled out among healthy participants (2). Even if patients were recruited from among early diagnosed patients, there are difficulties in assuring the homogeneity of the leprosy regarding age at infection, onset of clinical disease, and clinical and histopathologic diagnosis criteria in leprosy studies (31). Furthermore, in order to check the specificity of the antibody response among healthy controls, all seropositive samples were re-tested after adsorption with lepromin antigen, and 70% of the samples maintained the previous positive results (data not shown).
In our study, leprosy patients, patients with reactions, and healthy controls all had a low IgG anti-PGL-I antibody response, although there was variability in antibody concentrations. A previous study showed enhanced response of IgG anti-PGL-I antibodies in leprosy patients during onset and clinical remission of type I and type 2 reactions (30). However, the comparison with our study is difficult for many reasons: different antigen preparations were used, the small group of leprosy patients studied, and the absence of a healthy control group to determine the baseline antibody activity. IgG subtypes are influenced by different Thl/Th2 cytokines; however, recent studies in human leprosy (14, 16, 18) have not demonstrated a direct regulation of IgG subclasses by Thl/Th2 cytokines. PGL-I belongs to the category of glycolipids which were considered to be T-cell independent until the demonstration of the existence of the newly described CD8-/CD4-αβ+ T cells that are activated by nonprotein mycobacterial lipid and glycolipid antigens associated with the CD1 molecule (22).
Substantial progress has been made in eliminating leprosy as a public health problem, but there is still little progress in tools for predicting patients' reactions, a major cause of leprosy disability and a burden for the health service. The major challenge remains in applying the knowledge of the new molecular biology advances combined with the clinical, serological assays, and skin tests in order to build up information about the inflammatory process so that predicting reactions may ultimately be used in a public health context.
Acknowledgment. This work was partially sponsored by the Pan American Health Organization and Fundação de Apoio a Pesquisa da Universidade Federal de Goiás. We thank Dr. M. J. Colston for the PGL-I antigen and Dr. Richard Truman for the reagents and helpful suggestions in the early draft of the paper. We also thank Dr. lima M. Silva for clinical evaluation of the leprosy patients and lor the support of the Leprosy Control Program from the Brazilian Ministry of Health.
REFERENCES
1. BACH, M. A., WALLACH, D., FLAGUEL, B., HOFFENBACH, A. and COTTENTOT, A. Antibodies to phenolic glycolipid-I in leprosy patients-evolution under chemotherapy. Int. J. Lepr. 54 (1986) 256-267.
2. BAUMGART, K. W., BRITTON, W. J., MULLINS, R. J., BASTEN, A. and ARNETSON, R. C. Subclinical infection with Mycobacterium leprae -a problem for leprosy control strategies. Trans. R. Soc. Trop. Med. Hyg. 87 (1993) 412-415.
3. BRENNAN, P. J. and BARROW, W. W. Evidence for species-specific lipid antigen in Mycobacterium leprae. Int. J. Lepr. 48 (1980) 382-387.
4. BRETT, S. J., PAYNE, E. N., GIGG, J., BURGESS, P. and GIGG, R. Use of synthetic glycoconjugates containing the Mycobacterium leprae specific and immunodominant epitope of phenolic glycolipid-I in the serology of leprosy. Clin. Exp. Immunol. 64 (1986) 476-483.
5. BURGESS, P. J., FINE:. P. E. M., PONNIGHAUS, J. M. and DRAPER, C. Serological tests in leprosy; the sensitivity, specificity and predictive value of ELISA tests based on phenolic glycolipid antigens, and the implications for their use in epidemiological studies. Epidemiol. Infect. 101 (1988) 159-171.
6. CHO, S.-N., YANAGIHARA, D. L., HUNTER, S. W., GELHER, R. W. and BRENNAN, P. J. Serological specificity of phenolic glycolipid-I from Mycobacterium leprae and use in serodiagnosis of leprosy. Infect. Immun. 41 (1983) 1077-1083.
7. CHOUDHURI, K. The immunology of leprosy; unraveling an enigma. Int. J. Lepr. 63 (1995) 430-447.
8. FINE, P. E. M., PONNIGHAUS, J. M., BURGESS, P., CLARKSON. J. A. and DRAPER, C. C. Seroepidemiological studies of leprosy in northern Malawi based on an enzyme-linked immunosorbent assay using synthetic glycoconjugate antigen. Int. J. Lepr. 56 (1988) 243-254.
9. FINE, P. E. M.. STERNE, J. A. C. PONNIGHAUS, J. M. and REES, R. J. W. Delayed-type hypersensitivity, mycobacterial vaccines and protective immunity. Lancet 344(1994) 1245-1249.
10. FOSS. N. T., CALLERA, F. and ALBERTO, F. L. Anti-PGL-I levels in leprosy patients and their contacts. Bras. J. Med. Biol. Res. 26 (1993) 43-51.
11. GONZÁLEZ-ABREU, E., PON, J. A., HERNANDEZ, P., RODRIGUEZ, J., MENDOZA, E.. HERNANDEZ, M., CUEVAS, E. and GONZALEZ, A. B. Serological reactivity to a synthetic analog of phenolic glycolipid-I and early detection of leprosy in an area of low endemicity. Lepr. Rev. 67 (1996) 4-12.
12. HENNECKENS. J. L. and BURING, J. E:. Epidemiology in Medicine. 5th ed. Boston: Little, Brown and Company, 1987.
13. HOAGLIN, D. C, MOSTELLER. F. and TUKEY, J. W. Understanding Robust and Exploratory Data Analysis. New York: John Wiley & Sons, 1983.
14. HUSSAIN. R., KlFAYET, A. and CHIANG. T. J. Immunoglobulin Gl (IgGI) and IgG3 antibodies are markers of progressive disease in leprosy. Infect. Immun. 63 (1995) 410-415.
15. KAUFMANN, S. H. E. Immunity to intracellular bacteria. In: Fundamental Immunology. Paul, W. E., ed. New York: Raven Press Ltd., 1993. pp. 1251-1286.
16. KlFAYET, A. and HUSSAIN, R. IgG subclasses recognition pattern in leprosy: recognition of M. leprae antigen by IgG 1 and IgG3 antibodies is distinct across the disease spectrum. Int. J. Lepr. 64 (1996) 69-78.
17. KLATSER. P. R. Serology of leprosy. Trop. Geogr. Med. 46 (1994) 115-118.
18. KLATSER. P. R., JANSON, A. M., THOLE. J. E. R., BUHRER, S.. BOS, C. SOEBONO, H. and DE VRIES. R. R. P. Humoral and cellular immune reactivity to recombinant M. leprae antigens in HLA-typed leprosy patients and healthy controls. Int. J. Lepr. 65 (1997) 178-189.
19. LIENIIARDT, C. and FINE:, P. E. M. Type 1 reaction, neuritis and disability in leprosy. What is the current epidemiological situation? Lepr. Rev. 65 (1994) 9-33.
20. MARTELLI, C. M. T. MORALS NETO, O. L.. ANDRADE, A. L. S. S.. SILVA. S. A.. SILVA. I. M. and ZICKER. F. Spatial patterns of leprosy in an urban area in central Brazil. Bull. WHO 73 (1995) 315-319.
21. MEEKER. H. C, SCHULLER-LEVIS. G.. FUSCO, F., GIARDLNA-BECKET, M. A.. SERSEN, E. and LEVIS, W. R. Sequential monitoring of leprosy patients with serum antibody levels to phenolic glycolipid-I, a synthetic analog of phenolic glycolipid-1 and mycobacterial lipoarabinomannan. Int. J. Lepr. 58 (1990) 503-511.
22. MOODY. D. D, SUGITA, M., PETERS. P. J., BRENNER, M. B. and PORCELLI, S. A. The CD1 restricted T-cell response to mycobacteria. Res. Immunol. 147 Suppl. (1996) 550-559.
23. NOORDEEN, S. K. Eliminating leprosy as a public health problem; why the optimism is justified. Int. J. Lepr. 63 (1995) 559-566.
24. OTTENHOFF, T. H. M. Immunology of leprosy: lessons from and for leprosy. Int. J. Lepr. 62 (1994) 108-121.
25. REED, G. F, MEADE, B. D. and STEINHOFF, M. C. The reverse cumulative distribution plot: a graphic method for exploratory analysis of antibody data. Pediatrics 96 (1995) 600-603.
26. RIDLEY, D. S. and JOPLING, W. H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34 (1966) 255-273.
27. ROCHE, P. W., LE MASTER, J. and BUTLIN, R. Risk factors for type 1 reactions in leprosy. Int. J. Lepr. 65 (1997) 450-455.
28. ROCHE, P. W.. THEUVENET, J. W. and BRITTON, W. J. Risk factors for type I reactions in borderline leprosy patients. Lancet 338 (1991) 654-657.
29. SAAD, M. H. F., MEDEIROS, M. A., GALLO, M. E. N. and FONSECA, L. S. Use of the anti-PGL-I antibody ELISA and the Mitsuda reaction in early diagnosis of leprosy. Bras. J. Med. Biol. Res. 24 (1991) 801-805.
30. SAHA. K., CHATTOPADHYA, D., KASHYUP, A., AGARWAL, U. and CHAKRABARTY, S. K. Enhanced response of serum IgG class of anti-PGL-I antibodies in leprosy patients during onset and following clinical remission of type 1 and type 2 reactions. Int. J. Lepr. 63 (1995) 105-109.
31. SCOLLARD, D. M. Time and change: new dimensions in the immunopathologic spectrum of leprosy. Ann. Soc. Bclg. Med. Trop. 73 Suppl. 1 (1993) 5-11.
32. ULRICH, M., SMITH, P. G.. SAMPSON, C, ZUNIGA, M., CENTENO, M.. GARCIA, V., MANRIQUE, X., SALGADO, A. and CONVIT, J. IgM antibodies to native phenolic glycolipid-I in contacts of leprosy patients in Venezuela: epidemiological observations and a prospective study of the risk of leprosy. Int. J. Lepr. 59 (1991) 405-415 .
33. VAN BRAKEL. W. H.. KHAWAS. I. B. and LUCAS, S. B. Reactions in leprosy: an epidemiological study of 386 patients in west Nepal. Lepr. Rev. 65 (1994) 190-203.
34. YAMAMURA, M.. UYEMURA, K., DEANS, R. J., WEINBERG, K., REA, T. H., BLOOM. B. R. and MODI.IN, R. L. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science 254 (1991) 277-279.
1. M.M.A. Stefani. Ph.D.. Department of Immunology.
2. C. M. T. Martelli. M.D.. Ph.D.
3. O. I. Morais-Neto. M.D.. M.Sc. Department of Community Health.
4. P. Martelli. Research Assistant.
5. M. B. Costa. M.D.. Pathology Department. University Hospital. Federal University of Goiás. Goiânia. Goias, Brazil.
6. A.L.S.S. de Andrade. M.D., Ph.D., Communicable Disease Program. Pan American Health Organi/ation-WHO. Washington. DC . U.S.A.
Reprint requests to Prof. Mariane M. A. Stefani. Rua Delenda Rezende de Mello s/n Setor Universitario. 74605-050 Goiânia. Goiás, Brasil or FAX 55-62-202-3066. email = stefani@ internetional.com.br
Received for publication on 20 February 1998.
Accepted lor publication in revised forni on 10 July 1998.