- Volume 66 , Number 3
- Page: 365–73
Mycobacterium lepraemurium, a well-adapted parasite of macrophages: I. oxygen metabolites
ABSTRACT
We measured the release of reactive oxygen intermediaries [ROI (hydrogen peroxide and superoxide anion)] by murine peritoneal macrophages challenged in vitro with Mycobacterium lepraemurium (MLM), complement-opsonized yeast, M. bovis BCG, M. phlei. or phorbol myristate acetate (PMA). We found that except for MLM, all of the other materials provoked the release of significant amounts of hydrogen peroxide and superoxide. MLM entered the macrophages without triggering their oxidative metabolism. Pre-infection of macrophages with MLM did not alter these cells' capacity to release the normal amounts of ROI in response to other microorganisms or PMA. Killing of MLM did not revert the macrophages' failure to release ROI upon ingestion of the microorganism, nor were macrophages able to produce these toxic metabolites when pre-incubated in the presence of murine gamma interferon (IFN-γ). MLM has several attributes that allow it to survive within macrophages: a) it is a nontoxigenic microorganism (it does not harm its host), b) it resists the harsh conditions of the intraphagolysosomal milieu (a property perhaps dependent on its thick lipidie envelope), and c) it penetrates the macrophages without triggering their oxidative response (thus avoiding the generation of the toxic intermediaries of oxygen). For these attributes (and others discussed in this paper), we recognize MLM as a highly evolved, well-adapted parasite of macrophages.In addition, the results of the present study prompted the analysis of the biochemical pathways used by MLM and M. bovis BCG to penetrate into their cellular hosts, a subject now under investigation in our laboratory.
RÉSUMÉ
Nous avons mesuré la production de radicara libres intermédiaires derives de l'oxygéne (RLIO; péroxyde d'hydrogéne et anion superoxyde) á la suite de l'exposition in vitro de macrophages péritonéaux de souris par Mycobacterium lepraemurium (MLM), des levures opsonisées, M. bovis BCG. M. phlei ou l'acé-tate myristate de phorbol (AMP). Nous avons observé que, à l'exception de Ml.M, tous les autres agents élicitaienl le relarguage de quantités significatives de peroxyde d'hydrogène et d'anion superoxyde. MLM est entré dans les macrophages sans déclencher leur métabolisme oxydatif. L'infection antérieure de macrophages par MLM n'a pas altéré leur capacité à relâcher une quantité adéquate de RLIO en réponse aux autres micro-organismes ou à l'AMP. L'inactivation de MLM n'a pas abrogé l'incapacité des macrophages à relâcher des RLIO à l'occasion de la phagocytose de ces micro-organismes. De même, des macrophages pré-incubés avec de I'interferon gamma (IFN-γ) d'origine murine étaient incapables de produire ces metabolites toxiuqcs. Plusieurs propriétés de MLM lui permettent de survivre à l'intérieur du macrophage: a) c'est un micro-organisme non-toxigénique (il ne lèse pas l'hôte), b) il résiste les conditions |inhospitalières] du millieu intra-phagolysosomial (un caractère qui dépend peut-être d'une enveloppe lipidique épaisse) et c) il entre dans le macrophage sans déclencher une réponse oxydative (c'est à dire sans la production d'intermédiaires toxiques de l'oxygène). Du fait de ces propriétés (et d'autres dicuttées dans cet article), nous considérons MLM comme un parasite très évolué, parfaitement adapté aux macrophages.De plus, les résultats de cette étude a incité à entreprendre l'analyse des voies biochimiques utilisées par MLM et M. bovis BCG lorsqu'elles pénètrent leurs hôtes cellulaires, une recherche maintenant en cours dans notre laboratoire.
RESUMEN
Medimos la liberación de intermediarios reactivos del oxígeno, ROÍ (peróxido de hidrógeno anión superóxido) por los macrófagos peritoncales de ratón estimulados in vitro con Mycobacterium lepraemurium (MLM), levaduras opsonizadas con complemento, M. bovis BCG, M. phlei. o con el éster forbólico del ácido mirístico (PMA). Encontramos que excepto MLM, todos los otros materiales provocaron la liberación de cantidades importantes de peróxido de hidrógeno y de anión superóxido. MLM penetró en los macrófagos sin despertar en ellos el metabolismo oxidativo. La preinfección de los macrófagos por MLM no alteró su capacidad para liberar cantidades normales de ROÍ en respuesta a otros microorganismos o a PMA. Los macrófagos tampoco produjeron ROÍ cuando se trataron con MLM muerto por calor o cuando se preincubaron con interferon gamma (IFNγ). MLM tiene varios atributos que le permiten sobrevivir dentro de los macrófagos: a) es un microorganismo no toxigénico (no daña a su huésped), b) resiste las inhóspitas condiciones del medio intrafagosomal (una propiedad quizá dependiente de su gruesa estructura lipídica), y c) penetra a los macrófagos sin despertar su respuesta oxidativa (evitando así la producción de los intermediarios tóxicos del oxígeno). Por estos atributos (y otros discutidos en este trabajo), reconocemos a MLM como un parásito de los macrófagos altamente evolucionado y bien adaptado a la vida intracelular.Los resultados del presente estudio motivaron además, el análisis de las vias bioquímicas usadas por MLM y por M. bovis BCG para penetrar en los macrófagos, un tema de investigación actual en el laboratorio.
Pathogenic mycobacteria have developed a series of strategies to protect themselves from the noxious effects of the antimicrobial mechanisms of the phagocytic cells. The pioneering work of Hart, et al. (13) showed that virulent Mycobacterium tuberculosis resists the intracellular environment because it is able to inhibit the fusion of lysosomes with the mycobacterial-containing phagosomes. Shortly after, Goren, et al. (11) found that the purified sulfatides of the mycobacteria were themselves capable of preventing the phagosome-lysosome fusion in cultured macrophages [when formed, phagolysosomes contain most of the microbicidal weapons of the phagocytes, including neutral and acid hydrolytic enzymes, reactive oxygen intermediaries (ROI), and the very toxic system of the myeloperoxidase]. Then, Myrvik's group (23) provided evidence that M. tuberculosis H37Rv but not M. tuberculosis H37Ra was able to disrupt the phagosomal wall and escape to the cytoplasm where it could live freely, far from the inhospitable intraphagosomal environment. McDonough, et al. (20) found that some of the escaped bacilli lodged not only in the cytoplasm but also within nonfused vesicles. This has also been shown to happen with living M. leprae (28). More recently, it has been shown that M. tuberculosis H37Rv and virulent M. avium modify their phagosomes, making them more comfortable compartments (6). These modified phagolysosomes lack the acidifying system of the proton-ATPase (30) and other components of the mature phagolysosome (4). Under these conditions, acid-activated hydrolases do not fully operate and the myeloperoxidase (MPO) system is also considerably inhibited (16).
Other pathogenic mycobacteria do not make use of any of the above mechanisms of escape, but they possess other properties that render them extremely resistant to the bactericidal mechanisms of the phagocytes. Such is the case with M. lepraemurium (MLM), the etiologic agent of murine leprosy; it not only resists the intraphagosomal environment but seems to require the harsh conditions of it in order to survive [M. Lepraemurium multiplies exclusively within phagolysosomes (2)].
The endocytosis of most microorganisms by neutrophils and macrophages is usually detected by the biochemical "sensors" of the cells which then respond to the phagocytic stimulus in several ways. Many biochemical changes occur but one such change is the triggering of the oxidative metabolism that gives rise to the production and release of the toxic ROI. The ROI (which include superoxide anion, hydrogen peroxide, hydroxyl radicals, oxygen singlets and halogenating complexes) are unstable molecules, highly reactive and thus very toxic. When discharged into the phagolysosomes these ROI are a primary cause of bacterial killing. Since it has been reported that M. lepraemurium not only resists but also proliferates within fused phagolysosomes (9), we have analyzed the possibility that this microorganism might gain entrance into the macrophages without being noticed by the cellular biochemical detectors linked to production of ROI. To our knowledge there are only two papers in the literature pointing in this direction (1, 21)and some reports of the same sort applicable to M. leprae (17, 22). However, (here are also papers that do not fully sustain the results of some of the above reports (14, 15, 19, 27). Thus, we see the possibility that a microorganism (MLM, for instance) could enter macrophages without triggering their oxidative metabolism, as a subject that still deserves examination.
MATERIALS AND METHODS
M. lepraemurium. For over 15 years we have been working with the Hawaiian strain of M. lepraemurium. This strain (an original gift from Dr. Y. T. Chang, National Institutes of Health, Bethesda, Maryland, U.S.A.) is maintained by periodical intraperitoneal infection of mice. At the time the animals show clinical evidence of advanced infection (usually from 4 to 6 months post-inoculation) they are sacrificed, and the infected organs (liver and spleen) excised and processed to isolate the bacilli. Isolation of the bacilli is carried out by combining the entire procedure described by Prabhakaran, et al. to isolate M. lepraemurium (25) and the Percoll step of Draper's method proposed for the isolation of M. leprae (8), in that order. Purified bacilli are washed with phosphate buffered saline (PBS; 0.01M phosphate buffer in 0.15 M NaCl, pH 7.4), suspended in PBS, and the suspension adjusted to contain a known number of particles per ml (20 x 108 for the present study). The suspension is centrifuged (6000 × g × 10 min) and the pelleted bacilli resuspended in tissue culture medium (TCM) to the original volume. Aliquots of this suspension are kept frozen at -20ºC until used, usually within 3 months.
M. bovis BCG. We used the vacunal strain of BCG prepared by the Ministry of Health of Mexico (Danish strain 1331). The vaccine from several ampules was suspended in PBS, pooled, adjusted to 20 × 108 bacilli per ml, centrifuged, resuspended in TCM to the original volume, divided into aliquots, and kept frozen at -20ºC until used.
Mice. Adult, albino, female, NIH mice were used as the source of macrophages.
Chemicals. Most chemicals were from Sigma Chemical Co., St. Louis, Missouri, U.S.A.; tissue culture media and supplements were from GIBCO Labs., Grand Island, New York, U.S.A. Exceptions are indicated.
Peritoneal cell elicitation. Accumulation of cells in the abdominal cavity was induced by the intraperitonal (i.p.) injection of one of the following (sterile) materials: phosphate buffer solution (0.15M NaCl with 0.01M phosphate, pH 7.4); light mineral oil, or thioglycollate broth (Difco Laboratories, Detroit, Michigan, U.S.A.). Each animal was injected with 1.0 ml of one of the above stimuli and 4 days later the cell exudates were collected as described below.
Cell harvesting. To collect the peritoneal exudates, mice were killed by chloroform inhalation, bled by heart punction, soap-washed, submerged in 75% ethanol for several seconds, and pin-fixed on ad hoc dissection boards. After removing the abdominal skin, 5.0 ml of Alsever's solution was injected i.p. and the animals were shaken to promote the washing of the peritoneum. Then the peritoneal fluid was collected, pooled with the peritoneal fluid collected from 6 to 10 other mice, and centrifuged at 250 x g x 5 min to pellet the cells. The pellet was then washed with fresh Alsever's solution (250 x g x 3 min), treated for 30 sec with 2 ml of 0.2% NaCl to lyse erythrocytes, then with 2 ml of 1.6% NaCl to restore isotonicity, diluted to 20 ml with Alsever's solution for a final wash, centrifuged as before (250 x g x 3 min), and suspended in 3 ml of RPMI-medium supplemented with essential and nonessential amino acids, 2 mM glutamine, gentamicin (25 µg per ml), and 10% fetal calf serum (this complete medium will be referred to as TCM). The cells were counted and the suspension adjusted to contain 20 x 106 cells per ml. Viability, as assessed by Trypan-blue exclusion, was from 90% to 95%.
Cell cultures. Four million cells per ml/well were cultured in 24-well plates (Costar Corp., Cambridge, Massachusetts, U.S.A.) for 24 hr (37ºC, 5% CO J. At this time, dead and nonadherent cells were removed and the medium replaced with warm, fresh TCM. Preliminary experiments with 1 to 5 million cells per well (using opsonized yeast as stimuli) indicated that 4 x 106 was the most appropriate number of cells for the quantification of ROI under the conditions of our assays (the absorbances of the colored-reaction products were read in a Shimadzu spectrophotometer, using cells of 1.0 ml).
Stimuli. Cultured macrophages were stimulated with complement-opsonized Bakers' yeast (Saccharomyces cerevisiae; 20 yeast per cell), mycobacteria (M. lepraemurium, M. bovis BCG or M. phlei; 50 bacilli per cell), or phorbol 12-myristate 13-acetate (PMA; 1 µg in 10 µl of dimethyl sulfoxide per 106 cells).
Hydrogen peroxide production. The 24-hr macrophage cultures, prepared as described above, were gently washed with phenol red-free Hanks' balanced salt solution (HBSS) to eliminate dead and nonadherent cells, and then the cell monolayers were incubated in 1.0 ml of phenol red solution (10 mM phosphate, 140 mM NaCl, 5.5 mM glucose, and 0.113 mM phenol red, pH 7.0) containing 0.1 mg (20 IU) of horseradish peroxidase (24). As before, the stimuli were suspended or diluted in the phenol red-peroxidase (PR-HRPO) solution at the desired concentration. Macrophages were cultured with the several stimuli for 90 min at 37ºC under 5% CO2:. Production of H2O2 ; was assessed by adding 50 µl of 1 N NaOH to each well, reading the absorbances of the resulting purple colors at 600 nm against a blank of PR-HRPO. Readings were transformed to nanomoles of H2O2 in reference to a calibration curve prepared to contain from 0 to 100 nmol of H2O2 per ml. The results are expressed as nmol/90 min/4 x 106cells.
Superoxide anion production. Superoxide anion (O2- ) was measured in cultures containing 4 x 106 cells per ml per well in 24-well culture plates. All of the experiments were set up in quadruplicate. Peritoneal cells (mostly macrophages) were cultured for 24 hr in RPMI-FCS and then the dead and nonadherent cells were removed by replacing the 24-hr culture medium with 1.0 ml of phenol red-free HBSS, containing 1.0 mg of cytochrome C. The stimuli were suspended or diluted in the cytochrome C solution at the required concentration. The stimulated macrophage cultures were incubated for 90 min at 37ºC under a 5% atmosphere of CO2, then the supernates were collected, cleared by a brief centrifugation (350 x g x 3 min), and read at 550 nm in a Shimadzu spectrophotometer. The concentration of O2 was calculated from the extinction coefficient for reduced cytochrome C, according to the formula: nmol O2- = [absorbance at 550 nm x 100]/6.3 (27). The results are expressed as nmol O2-/90 min/4 X 106cells.
Viable versus heat-killed MLM. A suspension of freshly isolated M. lepraemurium (MLM) with 20 x 108 bacilli per ml was prepared in PBS. One aliquot of this suspension was left undisturbed (viable MLM), and a second aliquot was heated in the autoclave at 15 pounds for 10 min (heat-killed MLM). Then the two aliquots were spun down (6000 x g x 10 min), and the pellets suspended in phenol red-peroxidase at the original volume. Macrophage cultures were set up as described before (4 x 106cells per well), and the cell monolayers individually challenged with 0.1 ml of each bacterial suspension (50 bacilli per cell). Production of hydrogen peroxide was assessed as described above.
Release of ROI by MLM-preinfected macrophages. Macrophage monolayers (4 x 106 cells per well) were infected with viable MLM (50 bacilli per cell) for 24 hr. Then the cultures (washed once with warm TCM to remove dead cells and extracellular bacilli) were challenged with viable MLM, yeast, or PMA in PR-HRPO at the concentrations already indicated to measure hydrogen peroxide release as described above.
Effect of murine recombinant gamma interferon (mrlFN-γ) on ROI production. Macrophage monolayers prepared with 4 million cells per well were cultured for 24 hr in 1.0 ml of: a) tissue culture medium (TCM, MEM-FCS) or b) TCM with mrlFN-γ (200 IU per 106 cells). At this time, the incubation medium was removed and replaced with cytochrome C in HBSS (for superoxide detection) or with phenol red-peroxidase (for hydrogen peroxide detection). Then, sets of four cultures were stimulated with plain TCM, yeast, BCG, PMA or MLM for 90 min. Measurement of superoxide and hydrogen peroxide was carried out as described above.
Statistical analysis of results. The statistical analysis of the results was performed by applying the Kruskal-Wallis test for paired samples involving a single variable.
RESULTS
Macrophage elicitation. Although the amount of elicited macrophages was comparably high with both thioglycollate broth and mineral oil, for the purpose of the present study the best eliciting agent was mineral oil. Thioglycollate-induced peritoneal cells produced significant but highly variable levels of hydrogen peroxide on stimulation with opsonized yeast. Production of hydrogen peroxide by macrophages elicited with mineral oil was also high but it was less variable. Preliminary studies with unelicited and oil-induced macrophages showed a comparable behavior of these cells with regard to their spontaneous and PMA-induced release of ROI. For these reasons, oil-induced macrophages were used in the rest of the experiments. PBS did not work as an eliciting agent and was not used.
Production of hydrogen peroxide by cultured macrophages. Figure 1 shows a representative result on the production of H2O2 by peritoneal macrophages in response to the stimulus with yeast (YST), M. bovis BCG (BCG), or MLM. In this experiment, macrophages produced significant amounts of H2O2 when stimulated with YST (35.36 ± 1.07 nmole per 4 x 106 cells) or BCG (24.04 ± 0.70), but they did not produce detectable amounts of the peroxide when stimulated with MLM. Although in other experiments, resting and MLM-stimulated macrophages produced similar trace amounts of the peroxide, production of H2O2 by MLM-stimulated macrophages was rarely higher than the amount produced by the nonstimulated cells.
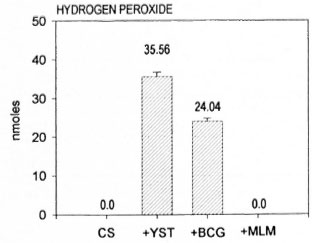
Fig. 1. Release of hydrogen peroxide by murine peritoneal macrophages stimulated in vitro with yeast(YST: 20 particles per cell), M. bovis BCG (BCG: 50 bacilli per cell) or M. lepraemurium (MLM; 50 bacilli per cell). Mean values ± S.E.M. (nmoles per 4 x 106cells per 90 min) are shown.
Production of O2- by cultured macrophages. As expected, cultured macrophages produced significant amounts of O2 when stimulated with YST (22.66 ± 1.15 nmole per 4 x 106 cells) or BCG (16.88 ± 0.38), but they did not respond to the stimulus with MLM (4.22 ± 0.38) better than the nonstimulated (resting) macrophages (4.66 ± 0.10 nmole). Figure 2 shows the results of a typical experiment.
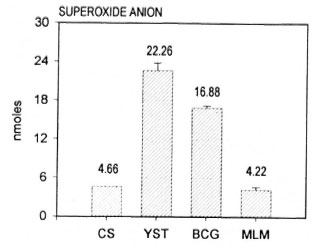
Fig. 2. Release of superoxide anion by murine peritoneal macrophages stimulated in vitro with yeast(YST: 20 particles per cell). M. bovis BCG (BCG; 50 bacilli per cell) or M. lepraemurium (MLM: 50 bacilli per cell). Mean values ± S.E.M. (nmoles per 4 x 106 cells per 90 min) are shown.
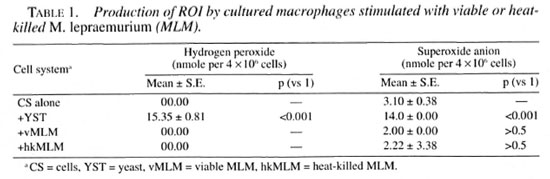
Production of ROI triggered by viable or nonviable MLM. Macrophages did not produce, or produced only trace amounts of ROI when stimulated with viable MLM. Heat-killing (15 pounds per 10 min) of the microorganism did not revert this result (Table 1).
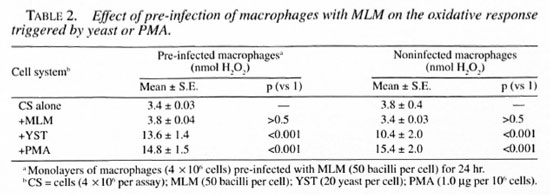
Release of ROI by macrophages preinfected with MLM. Macrophages preinfected for 24 hr with viable MLM remained unresponsive to MLM but responded normally to the challenge with yeast (YST) or PMA (Table 2).
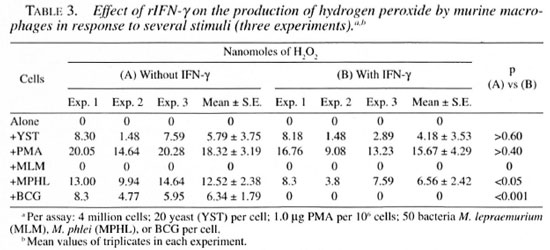
Release of ROI by macrophages preincubated with rIFN-γ. Macrophages pre-incubated for 24 hr with murine rlFN-γ did not improve their capacity to release hydrogen peroxide in response to yeast or PMA nor did they acquire the capacity to produce this metabolite in response to MLM. On the contrary, macrophages stimulated with M. phlei or BCG produced less hydrogen peroxide in the presence of rIFN-γ than in its absence (Table 3).
DISCUSSION
Macrophages and PMN leukocytes are the professional phagocytic cells of the body whose combined function accounts for the killing of invading microorganisms; killing by phagocytic cells depends in turn on a variety of mechanisms some of which are oxygen-dependent and some of which are not (26). Oxygen-dependent mechanisms include the generation of highly reactive oxygen species (free radicals) as well as the activity of the myeloperoxidase system. Among the oxygen-independent microbicidal mechanisms are: the acidic milieu within the phagolysosomes, the hydrolytic activity of neutral and acidic hydrolases, the effect of cationic proteins other than hydrolytic enzymes, and the activity of factors that increase the permeability of bacterial cell walls (BPIs). An additional microbicidal mechanism, this one strongly exerted by immunologically activated macrophages, depends on the metabolism of arginine in which nitric oxide is an important byproduct (12). As with oxygen metabolites (superoxide anion, hydroxyl radicals and oxygen singlet), nitric oxide is a highly unstable species and, thus, an extremely reactive radical. Oxygen metabolites and nitric oxide are able to chemically modify the microbial body, arresting its metabolism, leaving the microorganism inert, unable to repair itself, and exposed to the digestive action of the acid and neutral hydrolases found within the phagolysosome.
Stimulation of phagocytes via receptors to chemotactic factors or opsonins induces changes in the cell membrane whose signals are transduced to the cytoplasm and nucleus of the cell, leading both to specific and to more general biochemical changes. These changes are diverse but include increments in the cell activities related to phagocytosis (chemotaxis, endocytosis, and microbial killing) (17). Many microorganisms interact with receptors on the cell membrane in a manner regulated by G-proteins to next activate an effector molecule or second messenger (10). When the effector is an enzyme active on membrane inositolphospholipids (a phospholipase C, for instance), the hydrolyzing activity of the enzyme releases inositol triphosphate (IP3) and diacyl glycerol (DAG). While the role of IP, has much to do with Ca+ + mobilization through the opening of Ca+ + channels, DAG activates a type of protein kinase C (PKC) which then translocates to the inner part of the cell membrane to interact (in a particular situation) with the trimolecular complex of the NADPH-oxidase system (3, 7). This system, formed by the proteins p47, p67 and Cyt B558, participates in the trapping of molecular oxygen and in their chemical transformation into more reduced species. Oxygen is first reduced to superoxide anion (O2-) which is then transformed, either spontaneously or by the action of the enzyme superoxide dismutase (SOD), to hydrogen peroxide (H2O2). Superoxide anion may react with hydrogen peroxide to produce hydroxyl-radicals (HO·) or with extra molecules of superoxide anion to produce the so called oxygen singlet (1O2). Superoxide, hydrogen peroxide, hydroxyl radicals, and the oxygen singlets, constitute the oxygen-dependent microbicidal armory of phagocytic cells.
From the experiments shown in this paper it is clear that M. lepraemurium but not BCG behaves as a highly evolved parasite in the sense that it penetrates the macrophages without triggering their harmful oxidative metabolism. This might be a reason why MLM causes disease. M. bovis BCG and M. phlei, on the other hand, penetrate the cells through the classical endocytic pathway triggering, in so doing, the oxidative response and the consequent release of the toxic oxygen intermediaries. Very likely, these metabolites participate in the killing of these microorganisms and are at least partially responsible for the self-limited evolution of the diseases they may cause.
Our overall results are in line with those reported by Mor, et al. (21)and by Brett and Butler (1) with M. lepraemurium, although we did not find that heat-killing of MLM reverted the failure shown by the living microorganism (1). Also, as has been found with M. leprae (18), pre-infection of the macrophages with MLM does not interfere with the release of H2O2 triggered by other stimuli such as yeast or PMA. This means that MLM does not alter the pathways used by other microorganisms to gain entrance into the macrophages (results with yeast), nor modify the biochemical mechanisms responsible for the generation of ROI (results with PMA). Because these experiments were performed in macrophages infected for 24 hr with MLM, it is possible that longer times of pre-infection might have led to different results, since others have shown that macrophages infected for 3-5 days with M. leprae showed defective activation by IFN-γ and defective microbicidal activity, cytotoxicity for tumor target cells, superoxide production and surface Ia antigen expression (29).
Antimycobacterial immunity depends finally on the role of macrophages, but resting macrophages exhibit a limited microbicidal capacity. This mycobactericidal capacity, however, can be increased upon activation. In vivo, macrophages become activated through cytokines derived from helper T-lymphocytes. Within these cytokines, IFN-γ is recognized as a potent macrophage activator. Thus, theoretically, pretreatment of macrophages with recombinant mouse gamma interferon (rmlFN-γ) should increase these cells' capacity to destroy intracellular microorganisms, perhaps through the increased generation (and release) of ROI. Our results do not support the link between IFN-γ-induced macrophage activation and increased levels of ROI. Pre-incubation of the macrophages with rmlFN-γ did not reverse the inability of MLM to trigger the release of ROI. This has also been observed with M. leprae (18). In addition, the finding that pre-incubation of macrophages with rmlFN-γ for 24 hr considerably suppresses the release of ROI triggered by M. bovis BCG and M. phlei, suggests either an inhibition of some participant(s) within the NADPH system or a diminished expression of cell receptors for BCG and M. phlei. Both possibilities deserve further investigation.
Since MLM seems to be taken up by macrophages through mechanisms that bypass the endocytic pathways linked to the triggering of the oxidative metabolism of the cells, we are now investigating those pathways involved in the ingestion of MLM and M. bovis BCG by murine macrophages.
Acknowledgment. This study was partially supported by the Dirección de Estudios de Posgrado e Investigación del I.P.N. (Project DEPI 970347: "Radicales libres del oxígeno y el nitrógeno en la lepra mu-rina") and by the Consejo Nacional de Ciencia y Tecnología (Project: 26427-M "La lepra murina como modelo de estudio de la lepra humana"). O. Rojas-Espinosa and I. Estrada-García hold fellowships from I.P.N. (COFAA and EDD), and SNI, México; P. Arce-Paredes is a fellow of COFAA and EDD, I.P.N.
REFERENCES
1. BRETT, S. J. and BUTLER, R. Interactions of Mycobacterium lepraemurium with resident peritoneal macrophages: phagocytosis and stimulation of oxidative burst. Clin. Exp. Immunol. 71 (1988) 32-38.
2. BROWN, C. A.. DDRAPER, P. and HART, P. D. Mycobacteria and lysosomes: a paradox. Nature 221 (1969) 658-660.
3. CHRISTIANSEN, N. O. and BORREGAARD, N. Translocation of protein kinase C to subcellular fractions of human neutrophils. Scand. J. Immunol. 29 ( 1989) 409-416.
4. CLEMENS, D. L. and HORWITZ, M. A. Characterization of the Mycobacterium tuberculosis phagosome and evidence that phagosomal maturation is inhibited. J. Exp. Med. 181 (1995) 257-270.
5. CREE. I. A. and BECK. J. S. The influence of killed Mycobacterium leprae and other mycobacteria on opsonized yeast phagocytosis. Clin. Exp. Immunol. 64 (1986) 35-40.
6. CROWLE, A. J.. DAHL., R.. ROSS, E. and MAY, M. H. Evidence that vesicles containing living, virulent Mycobacterium tuberculosis or Mycobacterium avium in cultured human macrophages are not acidic. Infect. Immun. 59 (1991) 1823-1831.
7. DIETER, P. Relationship between intracellular pH changes, activation of protein kinase C and NADPH oxidase in macrophages. FEBS 298 (1992) 17-20.
8. DRAPER, P. Purification of Mycobacterium leprae. Report of the fifth meeting of the Scientific Working Group on the Immunology of Leprosy, Geneva, 24-26 June. 1980. TDR/IMMLEP-SWG 5/80.3.
9. DRAPER, P. and HART, P. D. Phagosomes, lysosomes and mycobacteria: cellular and microbial aspects. In: Mononuclear Phagocytes in Immunity. Infection and Pathology, van Furth, R.. ed. Oxford: Blackwell Scientific Publs.. 1975. pp. 575-594.
10. EXTON, J. H. Mechanisms of action of calcium-mobilizing agonists: some variations on a young theme. FASEB J. 2 (1988) 2670-2676.
11. GOREN, M. B., HART. P. D.. YOUNG, M. R. and ARMSTRONG. J. A. Prevention of phagosome-lysosome fusion in cultured macrophages by sulfatides of Mycobacterium tuberculosis. Proc. Nat. Acad. Sci. U.S.A. 73 (1976) 2510-2514.
12. GREEN, S. J., NANCY, C. A. and MELTZER, M. S. Cytokine-induced synthesis of nitrogen oxides in macrophages: a protective host response to Leishmania and other intracellular pathogens. J. Leuk. Biol. 50 (1991) 90-103.
13. HART, P. D.. ARMSTRONG, A., BROWN, C. A. and DRAPER. P. Ultrastructural study of the behavior of macrophages toward parasitic mycobacteria. Infect. Immun. 5 (1972) 803-807.
14. HOLZER, T. J., KIZLAITIS, L., VACHUI.A, M., WEAVER, C. W. and ANDERSEN, B. R. Human phagocytic cell responses to Mycobacterium leprae and Mycobacterium bovis bacillus Calmette-Guerin. J. Immunol. 141 (1988) 1701-1708.
15. HOLZER, T. J., NELSON, K. E., SCHAUF, V., CRISPIN. R. G. and ANDERSEN, B. R. Mycobacterium leprae fails to stimulate phagocytic cell superoxide anion generation. Infect. Immun. 51 (1986) 514-520.
16. JACKETT, P. S., ABER. V. R. and LOWRIE, D. B. The susceptibility of strains of Mycobacterium tuberculosis to catalase-mediated peroxidative killing. J. Gen. Microbiol. 121 (1980) 381-386.
17. JOHNSTON, R. B. and KITAGAWA, S. Molecular basis for the enhanced respiratory burst of activated macrophages. Federation Proc. 44 (1985) 2927- 2932.
18. LAUNOIS, P.. MAILLERE. B.. DIEYE, A., SARTHOU, J. L. and BACH, M.-A. Human phagocyte oxidative burst activation by BCG, M. leprae, and atypical mycobacteria: defective activation by M. leprae is not reversed by interferon γ. Cell. Immunol. 124 (1989) 168-174.
19. MAROLIA. J. and MAHADEVAN, P. R. Reactive oxygen intermediates inactivate Mycobacterium leprae in the phagocytes from human peripheral blood. Int. J. Lepr. 57 (1989) 483-491.
20. MCDONOUGH, K. A., KRESS, Y. and BLOOM, B. Pathogenesis of tuberculosis: interaction of Mycobacterium tuberculosis with macrophages. Infect. Immun. 61 (1993) 2763-2773.
21. MOR, N., GOREN, M. B. and PABST, M. J. Mycobacterium lepraeinurium activates macrophages but fails to trigger release of superoxide anion. J. Immunol. 140 (1988) 3956-3961.
22. MOURA, A. C. N., MODOLELL. M and MARIANO, M. Down-regulatory effect of Mycobacterium leprae cell wall lipids on phagocytosis, oxidative respiratory burst and tumour cell killing by mouse bone marrow derived macrophages. Scand. J. Immunol. 46 (1997) 500-505.
23. MYRVIK, Q. N.. LEAKE, E. S. and WRIGHT, M. J. Disruption of phagosomal membranes of normal alveolar macrophages by the H37Rv strain of Mycobacterium tuberculosis. Am. Rev. Respir. Dis. 129 (1984) 322-328.
24. PICK, E. and KEISARI, Y. A simple colorimetric method for the measurement of hydrogen peroxide produced by cells in culture. J. Immunol. Meth. 38 (1980) 161-163.
25. PRABHAKARAN, K., HARRIS, E. B. and KIRCHHEIMIR. W. F. Binding of 14C-labeled DOPA by Mycobacterium leprae in vivo. Int. J. Lepr. 44 (1976)58-64.
26. ROOT, R. K.. and COHEN, M. S. The microbicidal mechanisms of human neutrophils and eosinophils. Rev. Infect. Dis. 3 (1981) 565-598.
27. SHARP, A. K. and BANERJEE, D. K. Hydrogen peroxide and superoxide production by peripheral blood monocytes in leprosy. Clin. Exp. Immunol. 60 (1985)203-206.
28. SIBLEY, L. D., FRANZBLAU , S. G. and KRAHENBUHL, J. L. Intracellular fate of Mycobacterium leprae in normal and activated mouse macrophages. Infect. Immun. 55 (1987) 680-685.
29. SIBLEY, L. D. and KRAHENBUHL, J. L. Induction of unresponsiveness to gamma interferon in macrophages infected with Mycobacterium leprae. Infect. Immun. 56 (1988) 1912-1919.
30. STURGILL-KOSZYCKI, S., SCHLESINGER, P. H., CHARABORTY. P., HADDIX, P. L., COLLINS, H. L., FOX, A. K., ALLEN, R. D., GLUCK, S. L., HEUSER, J. and RUSSELL, D. G. Lack of acidification in Mycobacterium phagosomes produced by exclusion of the vesicular proton-ATPase. Science 263 (1994) 678-681.