- Volume 66 , Number 3
- Page: 309–15
Effectiveness of bacillus Calmette-Guerin (BCG) vaccination in the prevention of leprosy; a case-finding control study in nagpur, India
ABSTRACT
A hospital-based, pair-matched, case-control study was carried out at Government Medical College Hospital in Nagpur in central India to estimate the effectiveness of BCG vaccination in the prevention of leprosy. The study included 314 incidence cases of leprosy [diagnosed by World Health Organization (WHO) criteria] below the age of 32 years. Each case was pair matched with one control for age, sex and socioeconomic status. Controls were selected f rom subjects attending this hospital for conditions other than tuberculosis and leprosy. A significant protective association between BCG and leprosy was observed (OR 0.29, 95% CI 0.21-0.41). The vaccine effectiveness (VE) was estimated to be 71 % (95% CI 59-79). The BCG effectiveness against multibacillary and paucibacillary leprosy was 79% (95% CI 60-89) and 67% (95% CI 45-78), respectively. It was more effective during the first decade of life (VE 74%; 95% CI 38-90), among females (VE 82%; 95% CI 64-90), and in the lower socioeconomic strata (VE 75%; 95% CI 32-92). The prevented fraction was calculated to be 51% (95% CI 38-62). In conclusion, this study has identified a benericial role of BCG vaccination in the prevention of leprosy in central India.RÉSUMÉ
Une étude basée sur un hôpital, à partir de paires associant cas et contrôles, a été enterprise au Centre Hospitalier Universitair Gouvernemental de Nagpur, en Inde centrale, pour estimer l'efficacité de la vaccination par le BCT à prevenir la lèpre. L'étude a inclu 314 nouveaux cas de lèpre [diagnostiques selon les critères utilisés par l'Organisation Mondiale de la Santé (OMS)] de moins de 32 ans. Chaque cas a été comparé à un contrôle d'âge, de sexe et de statut socioéconomique similaire. Les contrôles ont été sélectionnés à partir de sujets hospitalisés pour des maladies autres que lèpre et tuberculose. Une association de type protection, statistiquement significative, a été observée entre le BCG et la lèpre [OR 0.29, intervalle de confiance à 95% (IC 95%) 0.21-0.41]. L'efficacité vaccinale (EV) fut estimée à 71% (IC 95% 59-79). L'efficacité du BCG contre la lèpre multibacillaire et paucibacillaire était de 79% (IC 95% 60-89) et 67% (IC 95% 45-78), respectivement. Elle était la plus convaincante durant les 10 premières années de vie (EV 74%; IC 95% 38-90), pour le genre féminin (EV 82%; IC 95% 64-90) et parmi les catégories socioéconomiques basses (EV 75%; IC 95% 32-92). La fraction protégée fut déterminée être 51% (IC 95% 38-62). Pour conclure, cette étude montre qu'il existe un effet bénéfique de la vaccination par le BCG pour prévenir l'apparition de la lèpre dans le région centrale de l'Inde.RESUMEN
Se realizó un estudio controlado para evaluar la eficiencia de la vacunación con BCG en la prevención contra la lepra, en el Hospital del Colegio de Medicina del Gobierno en Nagpur, India. El estudio incluyó 314 casos de lepra (diagnosticados según lose criterios de la Organización Mundial de la Salud, OMS) de menos de 32 años de edad. Cada caso fue "apareado" en edad, sexo y estado socioeconómico, con un caso control. Los controles fueron seleccionados de entre los sujetos atendidos en el hospital por problemas diferentes a la lepra o la tuberculosis. Se observó una significante asociación proectora entre el BCG y la lepra (OR 0.29, 95% CI 0.21-0.41). La efectividad de la vacuna (EV) fue del 71% (95% CI 59-79). La efectividad del BCG contra la lepra rnultibacilar y la paucibacilar fue del 79% (95% CI 60-89) y 67% (95% CI 45-78), respectivamente. Fue más efectiva durante la primera década de la vida (EV 74%; 95% CI 38-90), entre las mujeres (EV 82%; 95% CI 64-90). y en el estrato socioeconómico más bajo (EV 75%; 95% CI 32-92). Se calculó que la fracción protegida fue del 51% (9% CI 38-62). En conclusión, este estudio ha identificado un efecto benéfico de la vacuna BCG en la protección contra la lepra en la India Central.The effect of the bacillus Calmette Guerin (BCG) vaccine on mycobacterial diseases is still controversial despite the fact that it is one of the most widely used vaccines (8). BCG vaccines are generally rationalized and paid for with reference to protection against tuberculosis. However, it was noted in 1939 that BCG vaccination could induce a positive Mitsuda reaction, itself associated with cell-mediated immunity against leprosy (5). This observation was later confirmed by several workers (7) and led to a series of studies to evaluate the protective efficacy of BCG against leprosy. Findings of three studies (16, 17, 25) added to the evidence that BCG vaccine affords greater protection against leprosy than against tuberculosis. Several prospective trials have revealed its protective efficacy against leprosy with a degree of protection varying from 20% to 80% (4, 5, 12, 17, 20, 22, 25). A review of nine available case-control studies (1-3, 6, 9, 15, 16, 18, 24) of BCG vaccination and leprosy carried out around the world have demonstrated the effectiveness of BCG in the range of 20%-81%. However, of the total seven controlled trials and nine case-control studies evaluating the role of BCG in prevention of leprosy carried out around the world, two trials (4, 25) and one case-control study (15) are reported from India, which harbors more than one third of the leprosy cases in the world. Moreover, there is a wide variation of efficacy and effectiveness reported from different parts of the world (1~6, 9, 12, 15~18, 20, 22, 24, 25). Even more conflicting results were reported when subtypes of leprosy were considered (1, 6, 15, 17, 18). With this background and fortified by the fact that no comprehensive information is available on the role of BCG in the prevention of leprosy across the country, including central India, the present case-referent study was undertaken to estimate the effectiveness of BCG vaccination against leprosy in Nagpur in central India.
MATERIALS AND METHODS
Background. The government of India started the National Tuberculosis Control Programme (NTCP) in 1962. The program identified districts as the working units in which primary prevention, early detection, chemotherapy and case holding were the major activities. One of the primary prevention measures to which a lot of emphasis was given was BCG vaccination. During the initial phase of the program BCG was administered to a broader age range. However, since implementation of the Expanded Programme of Immunization in 1978 emphasis has been given to BCG vaccination of infants. The BCG vaccine prepared from the Danish 1331 strain in Guindy, Madras, is used in a dose of 0.1 ml intradermally (i.d.) (0.05 ml dose for newborns). The vaccine is given without prior testing with purified protein derivatives (23).
Design and setting. The present study was carried out as a pair-matched, hospital-based, case-control study at the Government Medical College Hospital, Nagpur, India. The study center is a tertiary care hospital with a separate Leprosy Control Unit.
Selection of cases. A total of 314 incident consecutive cases [diagnosed by WHO criteria (26)] reporting to Government Medical College Hospital, Nagpur, during the study period were recruited into the study. As suggested by WHO (26), an individual was regarded as having leprosy if he or she showed one of the following cardinal signs: hypopigmented or reddish skin lesion(s) with definite loss of sensation (damage to the peripheral nerves, as demonstrated by loss of sensation and weakness of the muscles of the hands, feet, or face); positive skin smears. When skin smears were not available or not dependable, more than five skin lesions were classified as multibacillary (MB) leprosy, and skin lesions up to five were considered as paucibacillary (PB) leprosy. When skin smears were available and dependable smear negative was considered as PB and smear positive as MB leprosy. Nerve damage involving only one nerve trunk was considered as PB, and the involvement of many nerve trunks was considered as MB leprosy. The diagnosis and classification were performed by a dermatologist co-investigator. All of the study subjects (including the cases) were aged 32 years or younger to take into account those born since the beginning of the NTCP (23) in 1962.
Selection of controls. Control subjects were selected randomly from the patients admitted to Government Medical College Hospital, Nagpur, for conditions other than leprosy/tuberculosis during the study period. The exclusion criteria for the controls were: a history suggestive of the manifestations of any form of leprosy/tuberculosis in the past, a recent family history of chemotherapy or chemoprophylaxis with isoniazid. One control was selected for each case. The controls were pair-matched for age (within 1 year of the age of the case), sex and socioeconomic status, recorded using the modified Kuppuswamy scale (13) of socioeconomic status (SES) classification, using occupation, education and per capita income as parameters. This is a five-point scale, with class 1 representing the highest socioeconomic (upper) and class V representing the lowest (lower) status. Classes II, III and IV are represented by upper middle, lower middle and upper lower SES, respectively.
Measurement of exposure. Evidence of BCG vaccination was determined by direct observation of a BCG scar at the insertion of deltoid muscle, immunization records if available, and information from the study subjects (or parents in case of children). Cases or controls with missing data or uncertain about BCG vaccination were excluded from the study. The measurement of exposure was, thus, carried out as per the guidelines given by Smith (21).
Statistical analysis. Crude odds ratios (OR) for the matched design were calculated as described by Greenberg and Ibrahim (10). The method described by Schlesselman (10) was used for calculating 95% confidence intervals (95% CI) for the OR. Subgroup analysis for matching variables was carried out separately. MULTLR statistical software was used to calculate the OR and their 95% CI by the conditional logistic regression method. The effectiveness of BCG vaccination was calculated by the formula (1 - OR) x 100%, where OR is the estimated odds ratio. The proportion of potential new cases that were prevented, the "prevented fraction," was determined according to the method of Miettinen (14). The statistical analysis was done using the MINITAB statistical package and dedicated Turbo C routines.
RESULTS
Table 1 describes the subjects by the study characteristics. A majority of the cases were males in the age group of 10-20 years from the upper lower (class IV) and lower (class V) classes of Kuppuswamy's SES scale. Because of the small number of study subjects in classes III, II and I of the SES, these groups were merged for further analysis. The study included 213 (67.8%) cases of PB leprosy and 101 (32.2%) cases of MB leprosy. The prevalence of exposure was 41.7% and 70.4% in cases and controls, respectively. Of the 131 exposed cases, 124 (95%) had a BCG scar and 7 (5%) did not but had immunization records certifying BCG vaccination. Similarly, of the 221 exposed controls 208 (94%) had a BCG scar and 13 (6%) did not but had BCG vaccination records available with them.

Table 2 describes the crude and conditional logistic regression estimates of the OR and their 95% CI. The significant protective association between BCG and leprosy is evident from this table. A subgroup analysis revealed that the OR were lower in the younger than 10 years age group and in females, but were not significantly different from the other subgroups. The different SES also did not differ significantly from each other with respect to the estimated OR. The OR estimates were lower for MB leprosy (0.21; 0.11-0.40) compared to PB leprosy (0.33; 0.22-0.55), but this difference also was not statistically significant.
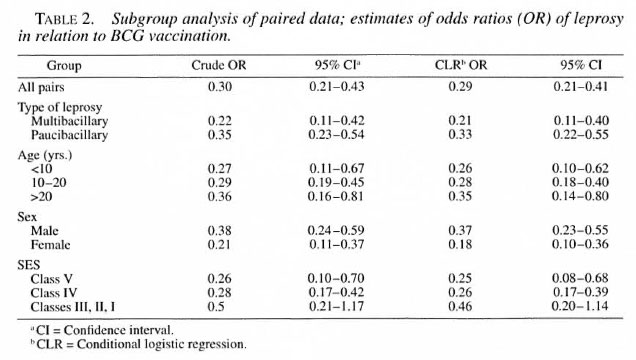
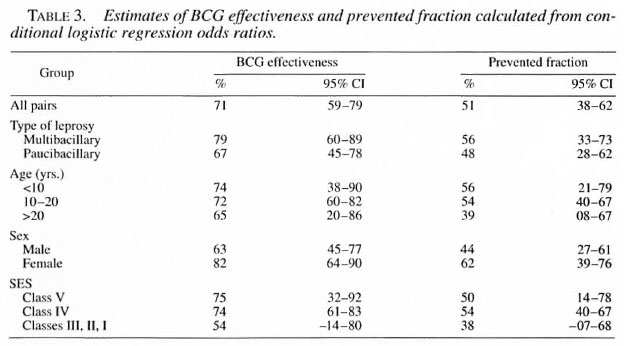
The protective effectiveness and prevented fraction were maximum for females, MB leprosy, those in the lower socioeconomic group, and subjects in the age group younger than 10 years (Table 3). BCG effectiveness and the prevented fraction for the whole study group were calculated to be 71 (59-79)% and 51 (38-62)%, respectively.
DISCUSSION
Age, sex and area of residence have conventionally been considered as the confounding factors. Most of the case-control studies, therefore, have used these as matching factors. Of these, however, area of residence has been recommended as a proxy for vaccination coverage rates. Because the present study was a hospitalized case-control study, the true population vaccination coverage rates would have been very difficult to estimate. Instead, we used another proxy measurement-socioeconomic status (SES). As described by Kue Young and Hershfield (11), these controls can be considered to be roughly equivalent to neighborhood controls. Moreover, the home address distribution of study subjects revealed that 96% of the cases and 92% of the controls came from Nagpur and adjacent districts.
The present study demonstrated a 12% excess protective effectiveness of BCG against MB leprosy as compared to PB leprosy, but this was not statistically significant. In the earlier matched case-control study carried out in western Kenya (16), the protection imparted by BCG against leprosy was estimated to be 81 % with no apparent difference in protection against PB and MB leprosy. However, effectiveness was slightly more (7%) against PB leprosy. A few more earlier studies (3, 6) have reported higher estimates of BCG effectiveness against MB leprosy compared to PB leprosy, which has significant public health implications because MB disease is thought to be a major source for the spread of Mycobacterium leprae in the community. However, these studies have found low estimates of BCG effectiveness against PB leprosy, contrary to the findings of the current study, but simultaneously argued that observed low protection can be attributed to the masking effect of study design, small sample size and other factors. Thus, in general, the findings of the present study are consistent with the theory that BCG vaccination brings about a shift in the immune response to a higher level of cell-mediated immunity and, thereby, offers protection, especially against the more severe MB form of the disease (3,15). When this phenomenon is operative, case-control studies on the protective effect of BCG vaccination on PB leprosy could suggest that there is no or less effect when, in fact, there is important effect, that is, BCG shifted the potential MB patients in the direction of the clinically less severe PB form of the disease.
The present study found the protective effect of BCG vaccination in the age group younger than 10 years to be higher than in the 10-20 years and >20 years age groups. Earlier studies have indicated that the protective efficacy of BCG vaccine increases with the interval of time since vaccination (16,24). However, another study from India (15) observed that with advancing age BCG effectiveness declines in females; this finding is somewhat similar to the observations of our current study.
The present study demonstrated 19% excess protective effectiveness of BCG vaccination in females as compared to males. This finding is in agreement with earlier reports (9, 15, 16). The only case-control study investigating the relationship between leprosy and BCG carried out in India (15) also has reported higher estimates of BCG vaccination in females. However, few case-referent studies(6, 24) have reported excess protective effectiveness of BCG vaccination in males. Although many studies, including the current study, have reported excess protective effectiveness of BCG vaccination in either sex, the differences were not statistically significant in a majority of these studies, including the present one.
With a major concentration of study subjects in the upper lower and lower socioeconomic classes, it is not surprising that BCG effectiveness is not significant in the other socioeconomic classes. The data indicate an absence of the hypothesized indirect effect of vaccination coverage through SES over BCG effectiveness. The finding has also been endorsed by a previous case-control study carried out in southern Vietnam (24).
The present study showed 71 (59-79)% vaccine effectiveness and 51 (38-62)% prevented fraction with use of BCG vaccination in the prevention of leprosy. This finding is in agreement with earlier studies (1, 3, 16, 18) which evaluated the role of BCG in the prevention of leprosy in other parts of the world. A review of nine available case-control studies and seven controlled trials of BCG vaccination and leprosy carried out around the world demonstrated the effectiveness of BCG in the range of 20%-81 %. The estimate of vaccine effectiveness (71%) observed in this study is relatively closer to the upper estimate of this range. Moreover, the proportion of potential new cases that were prevented, the "prevented fraction," was calculated to be 51 (38-62)%, i.e., the vaccination program prevented 51 out of every 100 cases that would have occurred in its absence. Results of this study, thus, indicated that BCG vaccination was effective against leprosy in central India.
Hence, in spite of the conflicting and controversial results of BCG effectiveness against tuberculosis (8) mass neonatal BCG vaccination has proved its utility and is justified in this population for the prevention of leprosy.
This finding is very encouraging for the prevention of a disease that has been more prevalent in India compared to other parts of the world. However, another case-control study carried out in South India (15) has reported relatively low estimates of BCG vaccine effectiveness in the prevention of leprosy. Hence, with this background and fortified by the fact that vaccine efficacy/effectiveness seems to be more dependent on geography and environmental factors than on vaccine strain, further studies should be carried out to evaluate the role of BCG in the prevention of leprosy in different parts of India.
REFERENCES
1. ABEL, L., CUA, V V., OBERTI, J,. LAP, V D., DUE, I. K., GROSSI, J. and LAGRANGE, P. H. Leprosy and BCG in southern Vietnam. (Letter). Lancet 335 (1990) 1536.
2. BAKER, D. M., NGUYEN-VAN-TAM, J. S. and SMITH, S. J. Protective efficacy of BCG vaccine against leprosy in southern Malawi. Epidemiol. Infect. 111 (1993) 21-25.
3. BOELENS, J. J., KROES, R., BEERS, S. V. and LEVER, P. Protective effect of BCG against leprosy in South Sulawesi, Indonesia. Int. J. Lepr. 63 (1995) 456-457.
4. CHAUDHURY, S., HAZRA, S. K., SAHA, B., MAZUMDER, B., BISWAS, P. C., CHATTOPADHYA, D. and SAHA, K. A eight-year field trial on antileprosy vaccines among high-risk household contacts in the Calcutta metropolis. Int. J. Lepr. 62 (1993)389-394.
5. CONVIT, J., SAMPSON, C., ZUNIGA, M., SMITH. P. G., PLATA, J., SILVA, J., MOLINA. J., PINARDI, M. E., BLOOM. B. R. and SALGADA, A. Immunoprophylactic trial with combined Mycobacterium leprae /BCG vaccine against leprosy; preliminary results. Lancet 339 (1992) 446-450.
6. CONVIT, J., SMITH, P. G., ZUNIGA, M., SAMPSON, C. ULRICH, M., PLATA, J. A., SILVA, J., MOLINA, J. and SALGADO, A. BCG vaccination protects against leprosy in Venezuela; a case-control study. Int. J. Lepr. 61 (1993) 185-191.
7. DOULL, J. A.. GUINTO, R. S. and MABALAY, M. C. Effect of BCG vaccination, lepromin testing and natural causes in inducing reactivity to lepromin and tuberculin. Int. J. Lepr. 25 (1957) 13-37.
8. FINE, P. E. M. BCG vaccination against tuberculosis and leprosy. Br. Med. Bull. 44 (1988) 691-703.
9. FINE. P. E. M., PONNIGHAUS, J. M., MAIN, N., CLARKSON. J. A. and BLISS. L. The protective efficacy of BCG against leprosy in northern Malawi. Lancet 2 (1986) 499-502.
10. GREENBERG, R. S. and IBRAHIM, M. A. The case-control study. In: Oxford Textbook of Public Health. Holland, W. W.. Detels, R. and Knox. G.. eds. London: Oxford University Press, 1985, pp. 123-143.
11. KUE; YOUNG, T. and HERSHFIELD, E. S. A case-control study to evaluate mass neonatal BCG vaccination among Canadian Indians. Am. J. Public Health 76(1986) 783-786.
12. LWIN. K.. SUNDARESAN, T, GYI, M. M., BECHELLI, L. M.. TAMONDONG, C., GARBAJOSA, P. G., SANSARRICQ, H. and NOORDEEN, S. K. BCG vaccination of children against leprosy: fourteen year findings of the trial in Burma. Bull. WHO 63 (1985) 1069-1078.
13. MAHAJAN, B. K. and GUPTA, M. C. Social environment. In: Textbook of Preventive and Social Medicine. New Delhi: Jaypee Bros., 1991, pp. 82-86.
14. MIETTINEN, O. S. Proportion of disease caused or prevented by a given exposure trait or intervention. Am. J. Epidemiol. 99 (1974) 325-332.
15. MULIYIL, J.. NELSON, K. E. and DIAMOND. E. L. Effect of BCG on the risk of leprosy in an endemic area; a case-control study. Int. J. Lepr. 59 (1991)229-236.
16. OREGE, P. A., FINE. P. E. M., LUCAS. S. B., OBURA, M., OKELA. C. and OKUKU. P. Case-control study of BCG vaccination as a risk factor for leprosy and tuberculosis in western Kenya. Int. J. Lepr. 61 (1993)542-549.
17. PONNIGHAUS, J. M., FINE, P. E. M., STERNE, J. A. C, WILSON, R. S., MSOSA, E., GRUER. P. J. K., JENKINS, P. A., LUCAS, S. B., LIOMBA, G. and BLISS, L. Efficacy of BCG against leprosy and tuberculosis in northern Malawi. Lancet 339 (1992) 636-639.
18. RODRIGUES, M. L. O., SILVA, S. A., NETO, J. C. A., DE ANDRADE, A. L. S. S., MARTELLI, C. M. T. and ZICKER, F. Protective effect of intradermal BCG against leprosy: a case-control study in central Brazil. Int. J. Lepr. 60 (1992 ) 335-339.
19. SCHLESSELMAN, J. J. Case-Control Studies: Design, Conduct, Analysis. New York: Oxford University Press. 1982 . pp. 171-226.
20. Scott, G. C, RUSSELL. D. A., BOUGHTON, C. R. and VINCIN, D. R. Untreated leprosy: probability of shifts in Ridley-Jopling classification; development of "Flares" or disappearance of clinically apparent disease. Int. J. Lepr. 44 (1978) 110-122.
21. SMITH, P. G. Retrospective assessment of the effectiveness of BCG vaccination against tuberculosis using the case-control method. Tubercle 62 (1982) 23-35.
22. STANLEY, S. J., HOWLAND, C, STONE, M. M. and SUTHERLAND, I. BCG vaccination of children against leprosy in Uganda; final results. J. Hyg. (Cambridge) 87 (1981 ) 233-248.
23. SURI, A. K. Notional Programme for Control of Tuberculosis. New Delhi: National Institute of Health and Family Welfare, 1988. pp. 11-34.
24. THUC, N. V, ABEL, L.. LAP, V. D.. OBERTI, J. and LAGRANGE, P.H,. Protective effect of BCG against leprosy and its subtypes; a case-control study in southern Vietnam. Int. J. Lepr. 62 (1994) 532-538.
25. TRIPATHY, S. P. The case for BCG. Ann. Natl. Acad. Med. Sci. (India) 19 (1983) 12-21.
26. WORLD HEALTH ORGANIZATION. A guide to eliminating leprosy as a public health problem. Geneva: World Health Organization, 1995 . pp. 15-26.
27. WUNSCH-FILHO, V., MONCAU, J. E. and NAKAO, N. Methodological considerations in case-control studies to evaluate BCG vaccine effectiveness. Int. J. Epidemiol. 22 (1993) 149-155.
1. Associate Professor. Clinical Epidemiology Unit and Department of Preventive and Social Medicine.
2. M.D., Lecturer. Department of Microbiology.
3. M.D., Lecturer, Leprosy Control Unit.
4. M.D.. Dip. Tuberculosis, Associate Professor and Head. Department of Tuberculosis and Chest Diseases.
5. M.D.. Clinical Epidemiology Unit and Dean. Government Medical College, Nagpur. India.
Reprint requests to Dr. S. P. Zodpey. 305 Hanuman Nagar. Nagpur 440 009. Maharashtra. India.
Received for publication on 11 December 1997.
Accepted lor publication in revised form on 16 July 1998.