- Volume 66 , Number 4
- Page: 464–74
Susceptibility to Mycobacterium leprae of ALY (Alymphoplasia) mice and IFN-γ induction in the culture supernatant of spleen cells
ABSTRACT
The alylaly (alymphoplasia) mice f rom a mutation of a colony of the C57BL/6J mouse strain, which has a systemic absence of lymph nodes and Peyer's patches, are deficient in both T- and B-cell-mediated immune functions. We have undertaken a comparison of susceptibility to Mycobacterium leprae of ALY (alylaly, aly/+) mice with C57BL/6J mice. The alylaly mouse was found to have an excellent high susceptibility to M. leprae with no distinction between female and male. The aly/+ mouse also was more susceptible to M. leprae at an earlier stage than the C57BL/6J mouse. Therefore, we examined and compared the cytokine gene expression and gamma interferon (IFN-γ) induction in the splenocytes of ALY mice. The expression of interleukin 4 (IL-4), IL-10 and IL-12 mRNA was weakly stimulated with ML-lysate in inoculated alylaly mice but IL-2, IL-6, IGIF/IL18 and IFN-γ mRNA were not observed. None of the cytokine genes used appeared, except the mRNA for IL-1-α, when uninfected cultured spleen cells were stimulated with ML-lysate. Also, IFN-γ production was not induced. However, the appearance of these cytokine genes was observed when stimulated with concanavalin A (ConA), and IFN-γ production was also induced in the culture supernatant by aly/+ and even alylaly mice stimulated with ConA. To examine the reason why IFN-γ cannot be produced by splenocytes of ALY mice inoculated with M. leprae, we detected cytokine gene expression and IFN-γ induction in the presence of recombinant murine IL-12 or IGIF/IL-18. IL-2 mRNA expression was detected in all of the mice tested in the presence of IL-12 but not in alylaly mice under IGIF/IL-18, and iNOS mRNA expression was not observed in alylaly mice under IL12 or IGIF/IL-18. IL-4 and IL-10 mRNA were detected by alylaly mice only by exposure to IGIF/IL-18. In culture, the supernatant with ML antigens of the alylaly mice did not produce IFN-γ in spite of the presence of IL-12 and IGIF/IL-18, while IFN-γ was weakly induced in aly/+ mice stimulated with ML-lysate and in the presence of IGIF/IL-18. Nevertheless, IFN-γ production was observed in splenocytes of the alylaly mice stimulated with ConA and also with IGIF/IL-18 plus anti-CD3 antibody. Our results suggest that ALY mice might be showing a high susceptibility to M. leprae because of deficient priming for activation of T cells with the leprosy bacilli infection. Moreover, it is possible that the phagocytic activities of the macrophages of ALY mice are also impaired.RÉSUMÉ
Les souris mutantes alylaly (alymphoplasia), issues d'une mutation à partir d'une colonie de souche C57BL/6J, présentent une absence généralisée de noeuds lymphatiques et de plaques de Péyer. Elles sont déficientes pour l'immunité médiée à la l'ois par les cullules B et T. Nous avons entrepris de comaparer la susceptibilité des souris ALY (alylaly, aly/+) à Mycobacterium leprae par rapport aux souris C57BL/6J. Les souris alylaly ont présenté une excellente susceptibilité envers M. leprae sans différence notable entre mâles et femelles. Les souris aly/+ étaient également plus sensibles à M. leprae et à un stade plus précoce que les souris C57BI./6J. Nous axons de ce fait examiné et comparé les niveaux d'expression des gènes des cytokines et l'induction de production d'interféron gamma (IFN-γ) par les splénocytes. Che/ les souris alylaly inoculées, l'expression de l'ARN-méssager de l'interleukine 4(11.-4). I'IL-l0 et I'IL-12 était faiblement stimulée par des lysats de M. leprae. Aucune stimulation n'a été observée concernant les ARN-m de IL-2. IL-6, IGIF/IL-18 et IFN-γ. Aucune expression génique de cytokine n'est apparue, à l'exception de I'IL-I-α, lorsque des cellules spléniques non infectées furent misent en culture et stimulées par un lysat de M. leprae. La production d'IFN-γ n'a pas non plus été induite. Cependant, l'apparition de ces gènes a été obtenue lorsque ces cellules furent stimulées par la concanavalinc A (ConA), et la production d'IFN-γ fut aussi induite dans le surnageant de cultures stimulées par la ConA chez les aly/+ et même les aly/aly. Afin d'examiner la raison pour laquelle IFN-γ n'est pas produite par les splénocytes provenant de souris ALY inoculées par M. leprae. nous avons étudié l'expression des gènes de cytokines et l'induction de IFN-γ en présence soit d'IL-12, soit d'IGIF/IL-18 recombinantes d'origine murine. L'expression de l'ARN messager de I'IL-2 était détectée chez toutes les souris testées en présence d'IL-12 mais pas chez les souris aly/aly sous IGIF-IL-18. et l'expression d l'ARN-m delà NO synthetase induite (iNOS) ne fut pas observée chez les souris aly/aly tant sous IL-12 que sous IGIF/IL-18. Les ARN-m de I'IL-4 et de I'IL-10 furent détectés par les souris seulement après exposition par IGIF/IL-18. En dépit de la présence d'IL-12 et d'IGIF/IL-18, les surnageants de cultures stimulées par des lysats de M. leprae provenant de souris alylaly ne contenaient pas d'IFN-γ. tandis que IFN-γ était faiblement induite chez les souris aly/+ stimulées par des lysats de M. leprae et en présence d'IGIF/IL-18. Néanmoins, la production de IFN-γétait observée dans les splénocytes de souris aly/aly stimulés par la ConA et par une combinaison de IGIF/IL-18 et d'anticorps anti-CD3. Nos résultats suggèrent que les souris ALY pourraient montrer une susceptibilité augmentée à A/. leprae liée à une déficience de priorité d'activation des cellules T par l'infection par la bacille de la lèpre. De plus, il est également possible que les capacités de phagocytose des souris ALY soient altérées.RESUMEN
Los ratones alylaly (alinfoplasia) derivados de una colonia de ratones C57BL/6J. tienen ausencia sistémica de ganglios linfáticos y de placas de Peyer. y son deficientes en inmunidad celular T y B. En este trabajo se compara la susceptibilidad a Mycobacterium leprae de los ratones ALY (alylaly y aly/+) con la susceptibilidad de los ratones C57BL/6J. Los ratones alylaly tienen una muy alta susceptibilidad a M. leprae, sin distinción entre hembras y machos. Los ratones aly/+ son también más susceptibles que los ratones C57BL/ 6J. En función de estas observaciones estudiamos la expresión de genes para diversas citocinas por los esplenocitos de los ratones ALY. La expresión de los mRNA para las interleucinas IL-4, II.-10. e 11.-12 en los esplenoeilos de los animales alylaly inoculados con M. leprae fue débilmente estimulada con un lisado de ML pero no se observó la expresión de mRNA para IL-2, IL-6, IGIF/IL-180 IFNγ. Cuando los esplenoeilos de animales no inoculados se estimularon con el lisado de ML no hubo expresión de mRNA para ninguna de estas citocinas. excepto prar IL-I-α. Sin embargo, los mRNA para todas estas citocinas se expresaron cuando los esplenocitos se estimularon con concanavalina A (ConA). El IFNγ se pudo detectaren los sobrenadantes de los esplenocitos alylaly y aly/+ estimulados con ConA. Para investigar porqué no se produce IFNγ por los esplenocitos de los ratones ALY inoculados con M. leprae. buscamos la expresión de genes para citocinas en presencia de IL-12 o IGIF/IL 18 recombinantes. La expresión del mRNA para IL-2 se detectó en todos los animales alylaly probados en presencia de IL-12 pero no en todos los casos probados en presencia de IGIF/IL-18; la expresión de mRNA para iNOS no se observó en ningún caso. Los mRNA para IL-4 e IL-10 en los esplenocitos alylaly sólo ocurrió en presencia de IGIF/IL-18. Los sobrenadantes de los cultivos de los esplenocitos alylaly no contuvieron IFNγ, no obstante la presencia de 1L-I2 e IGIF/IL-18. Hubo, en cambio, una débil inducción de IFNγ en los esplenocitos aly/+ estimulados con lisado de ML en presencia de IGIF/IL-18. La producción de IFNγ se observó en los esplenocitos alylaly estimulados con ConA y también con IGIF/IL-18, más anticuerpo anti-CD3. Nuestros resultados sugieren que la alta susceptibilidad de los ratones ALY a M. leprae puede deberse a una deficiente estimulación de las células T por el bacilo de la lepra. Fs probable que la actividad fagocítica de los macrófagos de los ratones ALY también esté alterada.
ALY (alymphoplasia) mice are due to an autosomal recessive mutation of the aly gene, and were established by Miyawaki, et al. (10,11). The mutant homozygous (alylaly) mouse, which has a systemic absence of lymph nodes and Peyer's patches, is deficient in both T- and B-cell-mediated immune functions. However, the thymus of the alylaly mouse has both subsets CD4 and CD8 just like the heterozygous (aly/+) mouse, but the overall cell count of T cells is higher than that in the aly/+ mouse when examined in the peripheral blood and spleen. The alylaly mice have a reduced capacity to mount Type IV immune reactions, [e.g., delayed-type hypersensitivity (DTH) reactions] and have a reduced capacity to form granulomas compared to aly/+ mice.
In this study, we compared the susceptibility to Mycobacterium leprae of ALY (alylaly, aly/+) mice with C57BL/6J mice. The study also attempts to compare the cytokine gene expression using the reverse transcriptase polymerase chain reaction (RT-PCR) method and gamma interferon (IFN-γ) production by an ELISA. We also studied the effect of interleukin 12 (IL-12) (6,18,19) and interferon gamma inducing factor (IGIF)/IL-18 (14) on IFN-γ induction in ALY and C57BL/6J mice.
MATERIALS AND METHODS
Mice. The mutant homozygous (alylaly), heterozygous (aly/+) and C57BL/6J mice were obtained from the Central Institute for Experimental Animals, Kawasaki, Japan. Eight l0-week-old, female and male mice were used for hind foot pad infection. Two age-matched female alylaly and aly/+ mice were used for spleen cells which were stimulated with M. leprae antigens for cytokine gene detection and IFN-γ induction. Two female C57BL/6J mice also were used for the effects of IL-12 and IGIF/IL-18 on cytokine gene expression and IFN-γ induction. The alylaly and aly/+ mice were maintained in a vinyl isolator under specific pathogen-free (SPF) conditions, and were provided with a sterilized, autoclavable commercial diet (CE-2; Clea Japan, Inc., Kawasaki, Japan), and tap water ad libitum. C57BL/6J control mice were housed in a conventional animal room.
M. leprae. M. leprae Thai-53 strain, derived from foot-pad passage of nude mice, were kindly provided by Dr. M. Matsuoka, Leprosy Research Center, National Institute of Infectious Diseases, Tokyo, Japan. The suspension was prepared and used as described previously (17).
Inoculation. The inoculum size was 1.8 x 107 bacilli/foot pad of both hind feet (BHF).
Harvests for M. leprae and histopathological studies. The mice were sacrificed 90 to 600 days after inoculation in order to confirm the multiplication and growth pattern of bacilli in the right hind foot pad, and the left hind foot pad was used for histopathological staining with hematoxylin and eosin (H&E) and Fite-Faraco stain. For identification, an immuno-histopathological stain with the avidin-biotin-peroxidase complex (ABC) method was also done to confirm the presence of phenolic glycolipid-I (PGL-I). For the dissemination of the infection, the other tissues were fixed with a 10% buffered formalin solution, processed for paraffin sections, and then stained with Fite-Faraco stain and H&E.
Cytokine gene detection by RT-PCR and IFN-γ induction. The expression of cytokine genes; IL-l-α, -2, -4, -6. -10, -12 (p40); IGIF/IL-18; IFN-γ; iNOS (inducible NOS); CD4, CD8 and β-actin in the cultured spleen cells was examined by the RT-PCR method. RT-PCR of cytokine mRNA in splenocytes from M. Leprae -infected mice 90 days after inoculation with age-matched uninoculated mice was performed as described (21).
Total RNA was isolated from spleen cells (1.0 x 106/well) cultured for 3 days in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS) and stimulated with M. leprae antigens [ML-lysate (lysate derived from M. leprae) (13) and ML-hsp65 (M. leprae heat-shock protein 65 derived from recombinant DNA) (12)]. Concanavalin A (ConA; Pharmacia Fine Chemicals, Piscataway, New Jersey, U.S.A.) was used and subjected to RT-PCR with specific primers as described previously (21).
Additionally, the primer sequences for IGIF/IL-18 and iNOS were as follows: IGIF/IL-18, 5'-ACTGTACAACCGCAGTAATACGG-3' and 5'-AGTGAACATTACAGATTTATCCC-3': iNOS. 5'-AAGTCAAATCCTACCAAAGTGA-3' and 5'-CCATAATACTGGTTGATGAACT-3'. The culture supernatants of the spleen cells stimulated with M. leprae antigens and ConA were collected and IFN-γ production was determined by an enzyme-linked immunosorbent assay (ELISA; Endogen, Cambridge, Massachusetts, U.S.A.).
Effects of IL-12 and IGIF/IL-18 on cytokine gene expression and IFN-γ induction. To confirm the effects of IL-12 and IGIF/IL-18, which are inducing factors for IFN-γ, recombinant murine IL-12 at Ing/ml per well (419-ML; R & D Systems. Minneapolis, Minnesota. U.S.A.) and recombinant murine IGIF/IL-18, derived from rDNA as mentioned by Okamura, et al. (14), at 1 U/ml per well were added to the spleen cell cultures (1.0 x 106/well) from uninfected alylaly, aly/+ and C57BL/6J mice. Total RNA was isolated from cultured spleen cells in the presence of IL-12 and IGIF/IL-IS and subjected to RT-PCR using the cytokine-specific primers as follows: IFN-γ, iNOS. IL-2, IL-4 and IL-10. The culture supernatants from the spleen cells in the presence of IL-12 and IGIF/IL-18, and also stimulated with ML antigens (ML-lysate, ML-hsp65), ConA and IGIF/ IL-18 plus rat anti-mouse CD3 (IgG) antibody (MCA 500G; Serotec, Oxford. U.K.) were assayed by an ELISA and compared for IFN-γ induction.
RESULTS
Susceptibility to M. leprae of ALY mice. The homozygous (alylaly) mice were highly susceptible to M. leprae. Bacillary counts exceeded the original inoculum at 90 days in alylaly mice. On day 150 after inoculation with M. leprae, almost all of the alylaly mice (which lack lymph nodes and Peyer's patches) showed a slight enlargement of the injected foot pads while the aly/+ and C57BL/6J mice did not show any enlargement. At 240 days post-inoculation, the swelling of the inoculated foot pads of alylaly mice had become more pronounced. The foot pad at that time was processed for Fite-Faraco's stain and microscopy showed numerous bacilli with mild lymphoid infiltrates (Fig. 1A). In the aly/+ mice, moderate amounts of bacilli are seen with more lymphoid infiltrates (Fig. 1B). The C57BL/6J mice had a scanty amount of M. leprae located at the blood vessel and nerve in the foot pad (Fig. 1C). At 360 days post-inoculation, there was a further increase of M. leprae in the foot pads of the alylaly mice (Fig. 2). The swelling of the inoculated foot pads measured 10 to 12 mm with a peacock dial thickness gauge. However, aly/+ mice showed a decrease in the number of M leprae in the inoculated foot pads after 360 days post-inoculation. The alylaly mice also showed systemic dissemination of M. leprae to the fore feet, lips and ears. In the male alylaly mice, the bacilli were seen in the scrotum and epididymis. Interestingly, the liver of alylaly mice showed many leprosy bacilli with a mild granuloma formation (Fig. 3) which was more marked at the central veins (Fig. 3, B and C) after 480 days post-inoculation. For identification of M. leprae, we used an immuno-histopathological stain with the ABC method and the presence of PGL-I was demonstrated at the site of multiplication of the bacilli.
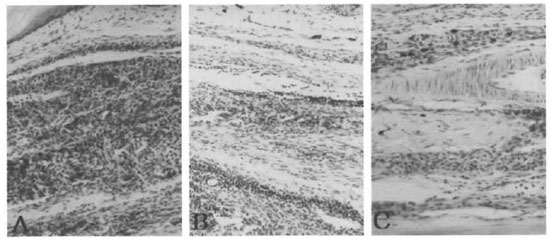
Fig. 1. Inoculated hind foot pads of AI.Y and C57BL/6J mice 240 days after inoculation with M. leprae . A= alylaly ; B = aly/+ ; C = C57BL/6J (File-Faraco x200).

Fig. 2. Enlargement of both inoculated hind foot pads of alylaly (alymphoplasia) mice 360 days after inoculation with M. Leprae . The alylaly mice of both sexes showed high susceptibility to M. leprae .
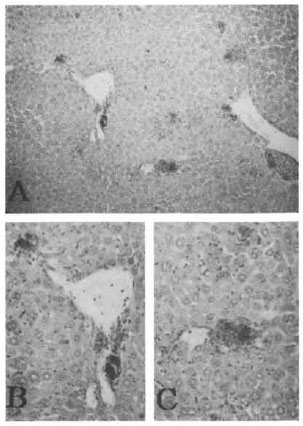
Fig. 3. Liver of an alylaly mouse 540 days after inuculation of both hind foot pads with M. leprae .Granuloma formation consisting of macrophages containing leprosy bacilli were seen in the liver, especially surrounding the central vein as shown in B and C (Fite-Faraco: A = x100; B and C = x200, high magnification of A).
Cytokine gene expression in cultured spleen cells from M. leprae-infected and age-matched uninoculated mice. The changes in cytokine gene expression in the cultured spleen cells stimulated with M. leprae antigens (ML-lysate, ML-hsp65) and ConA from alylaly and aly/+ mice uninoculated and inoculated with M. leprae at 90 days after inoculation are shown in Figure 4. There was no cytokine gene expression in the unstimulated cultured spleen cells of alylaly and aiyl+ uninoculated mice. Aside from the expression of CD4 and CD8, mRNA also were not seen in the cultured spleen cells of uninoculated mice. The appearance of CD8 mRNA was only induced in the cultured spleen cells of M. /V/;/w-infected mice but CD4 mRNA was never induced in the alylaly and aly/+ mice with or without stimulation. The expression of IL-2 mRNA was never seen in uninoculated mice stimulated with ML antigens but it was expressed weakly in the M. leprae inoculated aly/+ mice stimulated or not stimulated with ML antigens. However, it was never induced by cells from alylaly mice except for those stimulated with ConA. IL-4 mRNA was seen in the inoculated alylaly mice stimulated or not stimulated with ML antigens; whereas this gene was not expressed in aly/+ mice except for those stimulated with ConA. IL-6 mRNA was seen weakly in the inoculated mice stimulated with ML-hsp65 (Fig. 4, lanes 3 and 7), but it was never expressed in mice stimulated with ML-lysate. IL-10 mRNA expression was observed in inoculated mice stimulated with ML-hsp65 and ConA but was not expressed in inoculated mice stimulated with ML-lysate. The appearance of IFN-γ mRNA was induced in aly/+ mice stronger than in alylaly mice stimulated with ML-hsp65, but it was not induced in mice stimulated with ML-lysate while mice stimulated with ML antigens (uninoculated aly/+) and inoculated alylaly mice without stimulation showed IFN-γ mRNA. IGIF/IL18 mRNA expression was never induced in the cultured spleen cells of mice with or without M. leprae infection. IL-12 mRNA also was not expressed or appeared only weakly in inoculated alylaly mice but was not observed in aly/+ mice stimulated with ML antigens. More interestingly, the appearance of the cytokine genes, except for CD4 and IGIF/IL-18, was observed strongly in the inoculated mice stimulated with Con A (Fig. 4, lanes 4 and 8).
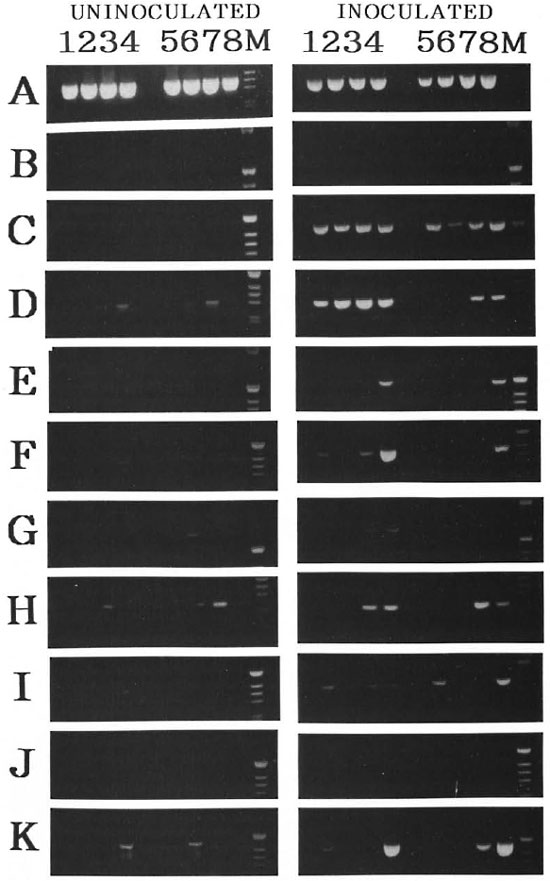
Fig. 4. Cytokine mRNA expression in cultured spleen cells of M. leprae -inoculated or uninoculated agematched ALY mice. RT-PCR analysis of cDNA from two spleen tissues pooled from two infected or uninfected age-matched ALY mice was carried out 90 days after inoculation with M. leprae . Reactions were incubated in a thermal cycler (Astec PC-800) for 35 cycles as conditions with denaturation 1 min, 94ºC or 95ºC; annealing 1 or 2 min. 55ºC. 60ºC or 65ºC: extension 1 or 3 min. 72ºC. Specific primers used were as follows: A = β-actin. B =CD4, C=CD8, D=IL-I-α, E=IL-2, F=IL-4. G=IL-6, H=IL-10, I= IL-12, J = IGIF and K = IFN-γ. Lanes 1 to 4 = splenocytes from alylaly mice: lanes 5 to 8 = splenocytes from aly/+ mice. Lanes 1 and 5 = controls (without antigen): Lanes 2 and 6 = stimulated with ML-lysate at 5 µg/ml: lanes 3 and 7 = stimulated with ML-hsp65 at 5 µg/ml; lanes 4 and 8 = stimulated with ConA at 5 µg/ml.
IFN-γ production in culture supernatant of spleen cells. IFN-γ production in the culture supernatant of the spleen cells after 3 days of stimulation with ML-lysate, ML-hsp65 and ConA was determined by an ELISA. IFN-γ production was not observed in the supernatant of the cultured spleen cells of the alylaly mice stimulated with ML-lysate and ML-hsp65; in those of the aly/+ mice stimulated with ML-lysate IFN-γ production was weakly seen. However, its induction was observed not only in the supernatant of inoculated but also in uninoculated alylaly and aly/+ mice stimulated with ConA (Fig. 5).
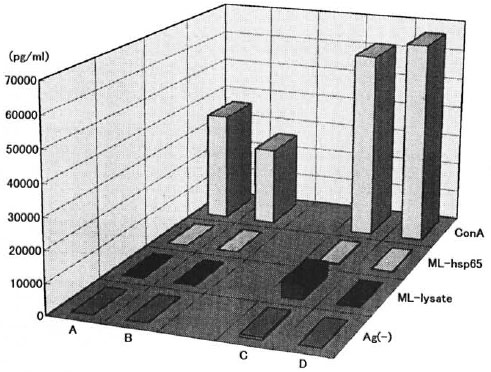
Fig. 5. IFN-γ production in culture supernatant of spleen cells from ALY mice with M-leprae -infection. Culture supernatant with stimulations from age-matched uninfected alylaly mice (A). M. leprae -infected alylaly mice (B), age-matched uninfected aly/+ mice (C) and M. leprae -infected aly/+ coice (D) were collected at 72 hr and assayed for IFN-γ by an ELISA.
Cytokine gene expression and IFN-γ induction in presence of IL-12 or IGIF/IL-18. We detected cytokine gene expression in the cultured spleen cells of alylaly, aly/+ and C57BL/6J uninfected mice in the presence of recombinant murine IL-12 and recombinant murine IGIF/IL-18. As seen in Figure 6, IFN-γ mRNA expression was induced by all of the mouse strains used in the presence of IL-12 and IGIF/IL-18. IL-2 mRNA expression was also detected and was stronger in the C57BL/6J mice than in the aly/+ mice in the presence of IL-12 or IGIF/IL-18. However, the alylaly mice never expressed IL-2 mRNA under IGIF/IL-18 but did express IL-2 mRNA under IL-12. iNOS and IL-4 mRNA expression were not detected or were weakly found in all of the examined mice in the presence of IL-12. However. iNOS mRNA was expressed under IGIF/IL-18 in C57BL/6J more strongly than in aly/+ mice but was not detected in alylaly mice. IL-4 mRNA was detected in alylaly as well as C57BL/6J mice in the presence of IGIF/IL-18. IL-10 mRNA was only detected in the alylaly mice in the presence of IGIF/IL-18 and was never detected under IL-12 in either of the ALY mice.
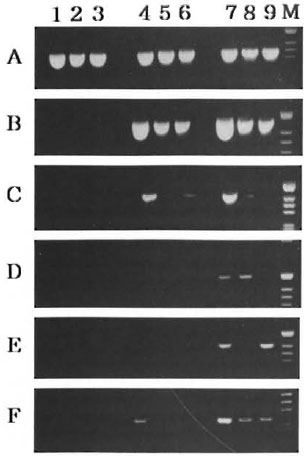
Fig. 6. Effects of IL-12 and IGIF/IL-18 on cytokine gene expression by ALY and C57BL/6J mice. RT-PCR analysis was done as in Figure 4 with specific primers of: β-actin (A), IFN-γ (B). IL-2 (C), iNOS (D), IL-4 (E) and IL-10 (F). Lane 1 (C57BL/6J), lane 2( aly/+ ) and lane 3 ( alylaly ) were splenocytes from controls (without cytokine). Lane 4 (C57BL/6J). lane 5( aly/+ ) and lane 6 ( alylaly ) were splenocytes by stimulation with IL-12. Lane 7 (C57BL/6J), lane 8 ( aly/+ ) and lane 9 ( alylaly ) were splenocytes by stimulation with IGIF/IL-18.
IFN-γ production in the presence of IL-12. IGIF/IL-18 only or with anti-CD3 antibody, and then stimulated with ML antigens or ConA, was examined by an ELISA. As seen in Figure 7, IFN-γ induction in the culture supernatant of spleen cells from alylaly mice was not found in spite of the presence of IL-12. while aly/+ mice induced IFN-γ more weakly than C57BL/6J mice under IGIF/IL-18 or ML-lysate. In this present study, we also were confirmed by the fact that the spleen cells stimulated with ML antigens by uninfected aly/aly mice did not show IFN-γ production in spite of the presence of IL-12 and IGIF/IL-18. However, IFN-γ production was observed in the culture supernatant of aly/aly mice stimulated with IGIF/IL-18 plus anti-CD3 antibody and also after stimulation with ConA (Fig. 5).
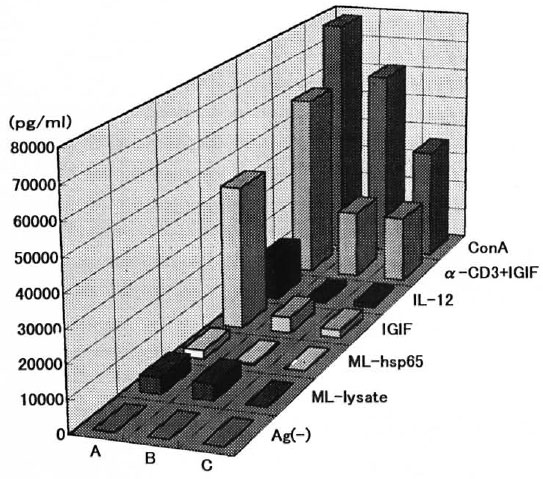
Fig. 7. Effects of IL-12 and IGIF/IL-18 on IFN-γ induction by ALY and C57BL/6J mice. Spleen cells from C57BL/6J mice (A), aly/+ mice (B) and alylaly mice (C) were stimulated with ML-lysate, ML-hsp65, IGIF/IL-18, IL-12, IGIF/IL-I8 plus anti-CD3 monoclonal antibodies and ConA. Culture supernatants were collected at 72 hr and assayed for IFN-γ by an ELISA.
DISCUSSION
We previously reported (20) the immunobiological characteristics of the congenital asplenic mouse which is deficient in B-cell immunity. This B-cell immunity defect is without any effect on the multiplication of M. leprae . Miyawaki, et al. (10,11) established the ALY (alymphoplasia) mouse with the gene symbol aly from a mutation of a colony of the C57BL/6J mouse strain. This mutant homozygous ( alylaly ) mouse is characterized by a systemic absence of lymph nodes and Peyer's patches and has a deficiency in both T- and B-cell immunity. A homozygous alylaly mouse is an interesting mutant mouse; the majority of thymocytes are Thy-l positive and all CD4/CD8 subsets are present in a proportion similar to the heterozygous aly/+ mouse which normally has lymph nodes and Peyer's patches. However, the ratio of B-to-T cells in the alylaly mice is lower compared to the aly/+ mice in the spleen and peripheral blood. The splenic cells of the alylaly mice proliferate in response to T-cell mitogens, ConA or PHA and a B-cell mitogen such as lipopolysaccharide. The delayed-type hypersensitivity (DTH) reaction and granuloma formation are likewise elicited on the alylaly mice (10,11).
In this present study, an attempt was made to demonstrate the influence of the above immunobiological characteristics of alylaly mice using aly/+ mice as controls on the growth of M. leprae following inoculation into both hind foot pads. The alylaly mouse was found to have an excellent high susceptibility to M. leprae (Fig. 1A). The aly/+ mouse (Fig. 1B) was also susceptible to M. leprae at an earlier stage of infection than the C57BL/6J mouse (Fig. 1C). On clay 360 following the foot-pad inoculation of M. leprae, further multiplication of leprosy bacilli and an enlargement in the infected foot pads of alylaly mice were observed with no distinction between females and males (Fig. 2). However, it was recognized that aly/+ mice showed a decrease in the number of M. leprae in the inoculated foot pads from day 240 to day 360. Based on these facts, aly/+ mice also are considered to be more impaired to some immunological functions, such as intracellular killing by macrophages for the eradication of M. leprae, than C57BL/6J mice. Therefore, we examined the cytokine gene expression in their cultured spleen cells stimulated with or without M. leprae antigens (ML-lysate. ML-hsp65) and also ConA by the RT-PCR method. Additionally, IFN-γ production was determined in the culture supernatant of their spleen cells by an ELISA. As shown in Figure 4, all of the cytokine genes tested did not appear physiologically in the cultured spleen cells of uninoculated ALY (alylaly, aly/+) mice (Fig. 4, lanes 1 and 5). their original background mice (C57BL/6J) (9) also never expressed any cytokine gene in their spleens while cytokine genes were found in the BALB/cAJcl mice (21). Interestingly, cytokine gene expression in the foot pads of C57BL/6J mice (9) was similar to that of BALB/cAJcl mice (21).
Inbred strains of mice are known to differ in their host reaction to mycobacteria or mycobacterial antigens (1,3,5,7). Our data suggest that the cytokine gene expression in the spleen may also have influence on the host reaction, such as DTH. T cells that express CD4 are generally helper/inducer cells, while those that express CD8 are usually cytotoxic cells (15). CD4/CD8 subsets are present in the homozygous alylaly mice in a proportion similar to the heterozygous aly/+ mice as described above. In infection with M. leprae in ALY mice, the appearance of CDS mRNA but not CD4 mRNA was induced in both the alylaly and aly/+ mice although it was not detected in the age-matched uninfected mice (Fig. 4). The expression of the IL-4, IL-10 and IL-12 mRNA was seen weakly in inoculated alylaly mice stimulated with ML-lysate; expression of IL-2, IL-6, IGIF/IL-18 and IFN-γ mRNA was not observed. None of the cytokine genes used (except for IL-I-α) in mice stimulated with ML-lysate were seen in uninfected cultured spleen cells. On the other hand, IFN-γ mRNA expression was seen in the uninfected aly/+ mice stimulated with ML antigens, and IL-2 mRNA expression was weakly induced in M. lep rae-inoculated mice.
The cytokine genes of IL-2, IFN-γ, IL-12 and IGIF/IL-18 (considered to work for host defense) (16) were not observed or were only weakly expressed by mice infected or stimulated with ML antigens. IFN-γ production was not seen or only weakly induced in the culture supernatant from spleen cells by ALY mice with ML-lysate or exposed to ConA (Fig. 5). The cultured spleen cells of ALY mice in the presence of ConA expressed all of the cytokine genes examined except for IGIF/IL-18 or CD4 mRNA. In addition, production of IFN-γ was induced in the culture supernatant from not only the aly/+ but also the alylaly mice. IFN-γ is a typical lymphokine, being produced exclusively by natural killer (NK) cells and the Thl subclass of CD4+ lymphocytes and certain CD8+ lymphocytes (2). IL-12 (6,18,19) and IGIF/IL-18 (14) are produced from activated macrophages and are known to induce IFN-γ due to augmentation of NK cell activity (15).
In order to examine the reason why IFN-γ cannot be produced in the culture supernatant of ALY mice with M. leprae infection but can be induced when stimulated with ConA, we tried to detect cytokine gene expression in the cultured splenocytes from uninfected alylaly, aly/+ and C57BL/6J mice in the presence of IL-12 or IGIF/IL-18. IFN-γ mRNA expression was induced by each of the strains used in the presence of IL-12. and similar results were observed with IGIF/IL-18. But other cytokine genes, such as IL-2, -4. -10 and iNOS mRNA. tested showed different patterns in the presence of IL-12 from the patterns with IGIF/IL-18. IL-2 mRNA expression was detected in all of the mice tested with IL-12 but not in the alylaly mice tested with IGIF/IL-18. iNOS expression was not observed in ALY mice with IL-12; however, this gene was observed in aly/+ mice, in C57BL/6J mice, but not in alylaly mice in the presence of IGIF/IL-18. In contrast, IL-4 and IL-10 mRNA were detected in the cultured splenocytes of alylaly mice which are deficient in both humoral and cell-mediated immune function in the presence of IGIF/ IL-18. However, iNOS, IL-4 and IL-10 mRNA were never expressed by either ALY mice while with C57BL/6J mice they were weakly observed in the presence of IL-I2. Manetti, et al. C) reported that T-cell lines (Thl) in the presence of IL-12 increased the production of IFN-γ, and exhibited a reduced ability to produce IL-4. IL-12 and IGIF/IL-18 may have different effects against host defense. On the other hand, in the culture supernatant with ML antigens alylaly mice did not produce IFN-γ and in spite of the presence of IL-12 and IGIF/IL-18, IFN-γ was only weakly induced in aly/+ mice stimulated with MLlysate and in the presence of IGIF/IL-18. Nevertheless, interestingly enough IFN-γ production was observed in alylaly mice stimulated with IGIF/IL-18 plus anti-CD3 antibody and also when stimulated with a T-cell activator such as ConA (Fig. 7).
Our results suggest that alylaly mice might be highly susceptible to M. leprae by the deficient priming for activation of the T cells with M. leprae. Moreover, it is possible that the phagocytic activities of the macrophages of ALY mice are also impaired.
Acknowledgment. We thank Mr. Kunio Kawatsu at the Leprosy Research Center. NIID, for the PGL-I. Also, we thank the Central Institute for Experimental Animals. Kanagawa. Japan, for the gill of ALY mice.
REFERENCES
1. ANDERSEN, P., LJUNGQVIST, L,. HASLOV, K., BENTZON, M. W. and HERON, L. Proliferative response to seven affinity purified mycobacterial antigens in eight strains of inbred mice. Int. J. Lepr. 59(1991)58-67.
2. BILLIAU, A. Interferon-γ: biology and role in pathogenesis. Adv. Immunol. 62(1996)61-130.
3. DOUGLAS-JONES, A. G. and WATSON, J. D. Immunity to leprosy. I. The proliferative response of murine T lymphocytes to Mycobacterium leprae. Int. J. Lepr. 53(1985)412-420.
4. DOUGLAS-JONES, A. G. and WATSON, J. D. Immunity to leprosy. II. Genetic control of murine T cell proliferative responses to Mycobacterium leprae. J. Immunol. 135(1985)2824-2828.
5. KAWAGUCHI, Y. and MATSUOKA, M. Observation of host reactions to murine leprosy bacilli in spread subcutaneous tissue preparations of various strains of mice. Jpn. J. Exp. Med. 47(1977)71-79.
6. KOBAYASHI, M., FITZ L,. RYAN, M., HEWICK, R. M., CLARK, S. C., CHAN. S., LOUDON, R., SHERMAN, F., PERUSSIA, B. and TRINCHIERI, G. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J. Exp. Med. 170(1989)827-845.
7. LOVIK, M., Haugen. O. A. and CLOSS, O. Delayed-type hypersensitivity after immunization with ultrasonicated Mycobacterium lepraemurium in C3H and C57BL mice. Scand. J. Immunol. 20(1984)227-235.
8. MANETTI, R., PARRONCHI, P., GIUDIZI, M. G., PICCINNI, M., MAGGI, E. TRINCHIERI, G. and TOMAGNANI, S. Natural killer cell stimulatory factor (interleukin 12 [IL-12]) induces T helper type 1 (Th1)-specific immune responses and inhibits the development of IL-4-producing Th cells. J. Exp. Med. 177(1993)1199-1204.
9. MATSUOKA, M. and YOGI, Y. [Development of delayed-type hypersensibilily and protective immunity in the mice sensitized with Mycobacterium leprae antigen.] Ann. Rept. U.S.-Japan Cooperative Medical Science Program, 1995, pp. 90-95.
10. MIYAWAKI, S. and NAKAMURA. Y. [Discovery of the aly mutant mouse with a general lack of lymph nodes accompanied by immunodeficiency]. Med. Immunol. 27(1994)453-456.
11. MIYAWAKI, S., NAKAMURA, Y. SUZUKA, H., KOBA, M., YASUMIZU, R., IKEHARI, S. and SHIBATA. Y. A new mutation, aly, that induces a generalized lack of lymph nodes accompanied by immunodeficiency in mice. Eur. J. Immunol. 24(1994)429-434.
12. NOMAGUCHI, H., MATSUOKA, M., KOHSAKA, K., NAKATA, A. and ITOH, T. Overproduction, affinity purification and characterization of 65-kDa protein of Mycobacterium leprae in Escherichia coli. Int. J. Lepr. 57(1989)817-824.
13. NOMAGUCHI, H., YOGI, Y. MATSUOKA, M., FUKUTOMI. Y, OKAMURA, H., NAGATA, K., NAGAI, S., OHARA. N. and YAMADA. T. [BCG vaccination to Mycobacterium leprae infection in mice.] Jpn. J. Lepr. 65(1996)106-112.
14. OKAMURA, H., TSUTSUI, H., KOMATSU, T. YUTSUDO, M., HAKURA, A., TANIMOTO, T., TORIGGOE, K., OKURA, T., NUKADA, Y, H.ATIORI, K., AKITA, K., NAMBA, M., TANABE, F., KONISHI, K., FUKUDA, S. and KURIMOTO, M. Cloning of a new cytokine that induces IFN-γ production by T cells. Nature 378(1995)88-91.
15. PARNES, J. R. Molecular biology and function of CD4 and CD8. Adv. Immunol. 44(1989)265-311.
16. ROITT, I., BROSTOFF, J. and MALE. D. Cell-mediated immune reactions. In: Immunology. 4th edn. Barcelona: Mosby (an imprint of Times Mirror International Publishers). 1996. pp. 9.1-9.15.
17. SHEPARD. C. C. Acid-fast bacilli in nasal excretion in leprosy, and results of inoculation of mice. Am. J. Hyg. 71(1960)147-157.
18. STERN, A. S., PODLASKI, F. J., HULMES, J. D., PAN. Y. E., QUINN. P. M., WOLITZKY, A. G., FAMILLETTI,P. C, STREMLO. L,. TRUITT, 'I'., CHIZZONITE., R. and GATELY. M. K. Purification to homogeneity and partial characterization of cytotoxic lymphocyte maturation factor from human B-lymphoblastoid cells. Proc. Natl. Acad. Sci. U.S.A. 87(1990)6808-6812.
19. WOLF, S. F., TEMPLE, P. A., KOBAYASHI, M., YOUNG, D., DICIG, M., LOWE, L., DZIALO, R., FITZ, L., FERENZ, C. HEWICK, R. M., KELLEHER, K., HERRMANN, S. H., CLARK. S. C. AZZONI, L,. CHAN, S. TRINCHIERI, F, and PERUSSIA, B. Cloning of cDNA for natural killer cell stimulatory factor, a heterodimeric cytokine with multiple biologic effects on T and natural killer cells. J. Immunol. 146(1991)3074-3081.
20. YOGI, Y. and NAKAMURA, K. The experimental inoculation with Mycobacterium leprae in the congenitally asplenic mouse. Jpn. J. Lepr. 55(1986)35-40.
21. YOGI, Y, NOMAGUCHI, H., SAKAMOTO, Y. MATSUOKA, M., FUJIMURA, T., OKAMURA, H., HIOKI, K., SAITO, M. and NOMURA. T. Cytokine gene expression in the foot pad and spleen of BALB/ cAJcl mice infected with M. leprae. Int. J. Lepr. 65(1997)80-89.
1. Ph.D.
2. Ph.D.
3. B.S., Tokyo Kiyose School for Medical Technology, 3-1-31 Umezono, Kiyose-shi. Tokyo 204. Japan.
4. Ph.D., Department of Bacteriology, Hyogo College of Medicine. Nishinomiya. Hyogo663, Japan.
5. Ph.D., Leprosy Research Center. National Institute of Infectious Diseases. 4-2-1 Aoba-cho. Higashimurayama-shi, Tokyo 189. Japan.
Received for publication on 3 November 1997.
Accepted for publication in revised form on 30 April 1998.