- Volume 66 , Number 4
- Page: 475–82
Anti-neutrophil cytoplasmic antibodies (ANCA) in the clinical forms of leprosy
ABSTRACT
Anti-neutrophil cytoplasmic antibodies (ANCA) are autoantibodies against enzymes present in primary granules of neutrophils and lysosomes of monocytes detected in systemic vasculitis and in other diseases, including infections. ANCA are markers of active Wegener granulomatosis, which presents some anatomo-pathologic and immune response features similar to those of leprosy. Thus, we raised the hypothesis that ANCA may be present in leprosy as markers specifically linked to the presence of vasculitis. The aim of this study was to determine the presence of ANCA in leprosy and its correlation with the clinical forms of the disease. Sera f rom 60 normal individuals and f rom 59 patients with different clinical forms of leprosy were, studied. The patients were also allocated into reactional and nonreactional groups. By indirect immunofluorescence, ANCA were positive, an atypical pattern (A-ANCA), in 28.8% of the patient sera. A-ANCA predominated, although not significantly (p > 0.05), in the reactional groups 37.9% vs 20.0%), and in those at the lepromatous pole (41.6% vs 20.0%). There was no correlation between ANCA positivity and either disease duration, disease activity, or therapeutic regimen (p > 0.05). An interesting finding was the correlation between ANCA and gender: 94.1% of ANCA-positive patients were males (p < 0.01), a feature that so far has not been reported in ANCA-related diseases and for which there is no explanation at the moment. By ELISA, the sera of the lepromatous leprosy patients did not show activity against either PR3, MPO, HLE, the most common ANCA antigens. Because A-ANCA are nonspecific, this finding requires further investigation for the determination of the responsible antigen(s). In conclusion, A-ANCA are present in 28.8% of leprosy patients but are not related to vasculitis in the erythema nodosum leprosum reaction and are not a marker of a specific clinicai form.RÉSUMÉ
Les anticorps anti-cytoplasmiques des neutrophiles (AACN) sont des auto-anticorps dirigés contre les enzymes des granules primaires des neutrophiles et des lysosomes des monocytes. Ils sont détectés au cours des vasculites systémiques et d'autres maladies, y compris les infections. Les AACN sont des markeurs de la phase active de la Granulomatose de Wegener. qui présente des caractères anatomo-pathologiques et immunologiques communs à ceux de la lèpre. De ce l'ait, nous avons proposé l'hypothèse que les AACN pourraient être présents dans les cas de lèpre comme marqueurs spécifiques liés à la présence de vasculites. Le but de cette élude était de déterminer si les AACN étaient presents dans les cas de lèpre et de leurs corrélations éventuelles avec les différentes formes cliniques de la maladie. Les sérums de 60 individus normaux et de 59 patients présentant des formes cliniques variées de la lèpre furent analysés. Les patients furent aussi divisés en groupe réactionnel et groupe nonréaclionnel. Les AACN furent positifs par immunofluorescence indirecte, avec une distribution atypique (A-AACN). chez 28.8% des sérums de patients. Les A-AACN étaient retrouvés plus fréquemment, de façon non stastiquement significative (p > 0.05), parmi le group réactionnel (37.9% contre 20%), et chez, les patients au pôle lépromateux (41.6% contre 20%). Il n'y avait pas de corrélation entre la positivité aux AACN et la duration de la maladie, ou le degré d'activité de la maladie, ou le protocole thérapeutique (p > 0.05). Une trouvaille intéressante fut la corrélation entre AACN et le sexe: 94.1% des patients positifs aux AACN sont des hommes (p < 0.01). un caractère qui n'a pas encore été rapporté dans les maladies associant des AACN et pour lequel il n'y a pas encore d'explication. Les sérums testés par ELISA provenant de patients présentant une lèpre lépromateuse n'ont pas présenté de positivité contre PR3, MPO et HLE, les antigènes contre lesquels les AACN sont les plus souvent dirigés. Comme les A-AACN sont non spécifiques, ces résultats demandent des études plus poussées pour déterminer quel(s) est (sont) le(s) antigène(s) responsable(s). En conclusion, des A-AACN sont présents chez. 28.8% de patients souffrant de lèpres mais ne sont ni la conséquence de vasculites associées aux réactions d'érythème noueux lépreux, ni le marqueur d'une forme clinique spécifique.RESUMEN
Los anticuerpos anticitoplásmicos (ANCA) son anticuerpos contra enzimas de los granulos primarios de neutrófilos y monocitos presentes en vasculitis sistémicas y otras enfermedades, incluyendo infecciones. Los ANCA son marcadores de la granulomatosis activa de Wegener, la cual presenta características anatomopatológicas e inmunológicas similares a las de la lepra. Propusimos la hipótesis de (pie los ANCA pueden estar presentes en la lepra como marcadores específicamente ligados a la presencia de vasculitis. En este esludio se determinó la presencia de ANCA en las diferentes formas clinicas de la enfermedad. Se estudiaron sueros de 60 individuos sanos y de 59 pacientes con lepra de diferente clasificación. Los pacientes se agruparon como casos reaccionales y como casos no reaccionales. Los ANCA fueron positivos por inmunofluorescencia indirecta (IFI) en 28.8% de los sueros probados. El patrón de IFI fue atípico (A-ANCA). Los anticuerpos A-ANCA predominaron, aunque no significativamente (p > 0.05). en los grupos reaccionales (37.9% vs 20.0%), y en los pacientes del extremo lepromatoso (41.6% vs 20.0%). No hubo correlación entre la positividad en ANCA y la duración de la enfermedad, la actividad de la misma, o el esquema de tratamiento (p > 0.05). Un hallazgo interesante fue la correlación entre ANCA y el género: 94.1% de los pacientes ANCA-positivos fueron hombres (p < 0.01) un hallazgo que hasta ahora no se ha reportado en enfermedades relacionadas con ANCA y para el cual no hay explicación por el momento. Por ELISA, los sueros de los pacientes lepromatosos no mostraron actividad contra PR3, MPO o ULE. los antigenos más comunes para ANCA. Debido a que los A-ANCA no son específicos, este hallazgo requiere de más investigación para identificar los antigenos involucrados. En conclusión, los anticuerpos A-ANCA están presentes en el 28.8% de los pacientes con lepra pero no están relacionados con vasculitis en la reacción tipo eritema nodoso leproso y no son exclusivos de ninguna forma clinica de le lepra.Anti-neutrophil cytoplasmic antibodies (ANCA) are immunoglobulins predominantly of the IgG class acting against the primary or azurophilic granules of neutrophils and monocyte lysosomes (33). Their known antigens are myeloperoxidase (MPO), proteinase-3 (PR-3), elastase (HLE), lactoferrin (LF), cathepsin G (CG), cationic protein (CAP 57), lysozyme, β-gluconidase, (α-enolase, bacterial membrane permeability increasing protein (BP1) and azurocidin (16).
Indirect immunofluorescence (IIF) is the test of choice for the screening of ANCA-positive sera, which reveals two classic patterns, i.e., the cytoplasmic (C-ANCA) and perinuclear (P-ANCA) patterns (16,29). The C-ANCA pattern is characterized by granular fluorescence in the cytoplasm of neutrophils, which is more marked among the lobules of the nucleus and is caused by the PR-3 or BPI antigen. The P-ANCA pattern is characterized by a concentration of fluorescence in the perinuclear region. The major antigen that gives origin to this pattern is MPO, but other enzymes such as HLE, CG and LF have been reported to be P-ANCA antigens (37). Other non-C and non-P fluorescence patterns are also commonly observed but no official nomenclature is available for them. They are generically called atypical (A-ANCA), and their clinical significance and the antigens involved are unknown (16,29).
ANCA are detected at varying frequencies in various types of systemic vasculitis and in other diseases, including infections (17,21,30). However, their clinical significance and their pathogenic role are still unknown. The only exception is Wegener's granulomatosis (WG) with which ANCA are clearly related, being used for diagnosis and monitoring of the response to therapy (33). WG is a systemic necrotizing vasculitis that especially involves the upper airways, lungs, kidneys and eyes. The first episode and/or recurrences are usually triggered by bacterial or viral infections (27). The pathogeny of WG has not been fully elucidated but genetic factors, infections, cell-immune response, endothelial cells, neutrophils and ANCA are known to be involved (20).
WG presents some anatomopathologic and immune response features similar to those of tuberculoid leprosy: both are chronic granulomatous diseases whose major mechanism of lesion involves the cell-immune system with a secondary role for the humoral-immune response. In addition, lepromatous leprosy, in which the humoral-immune response predominates, is directly linked to the development of vasculitis and erythema nodosum leprosum (ENL) (3,5,24-26)
On the basis of the similarities between the two diseases, we raised the hypothesis that ANCA may be involved in leprosy as a marker of the specific type linked to the presence of vasculitis. Thus, the objective of the present study was to determine the presence of ANCA in serum from leprosy patients and to correlate them with the clinical forms of the disease.
MATERIALS AND METHODS
Serum specimens from 59 patients with leprosy as diagnosed by experienced physicians at the Laura de Souza Lima Institute of Bauru in the state of Sao Paulo, Brazil, and without evidence of other concomitant diseases, mainly systemic lupus erythematosus, rheumatoid arthritis and systemic vasculitis, were studied. The cases of leprosy were classified according to the Congress of Madrid (19), and present two polar types, leproniatous and tuberculoid, and two groups, indeterminate and borderline. Because the cases in the borderline spectrum are unstable and tend to move in either direction, for convenience the borderline and indeterminate cases have been grouped under the term borderline leprosy. The cases considered for the study were both with and without reaction, who consented to undergo the investigation after the purpose of the study was explained to them. In reactional cases, blood for ANCA determination was drawn before the introduction of specific therapy. Normal controls consisted of 60 healthy blood donors registered at the Blood Bank of the Botucatu Medical School, Botucatu, Brazil.
Detection of ANCA by IIF
Testing for ANCA was done according to Wiik (36) as agreed in the First International Workshop on ANCA.
Granulocyte separation. Granulocytes were separated from blood of normal human donors using a Ficoll-Paque-density gradient (Pharmacia Biotech Inc,. Piscataway. New Jersey, U.S.A.). After red blood cell washing and lysis, the sediment was suspended in RPMI 1640 medium and the number of white blood cells adjusted to 1.0 x 106 cells/ml.
Slide preparation and fixation in ethanol. In each well of 12-well glass slides, 25 µl of the cell suspension was placed and incubated for 8 min in a humid chamber. After incubation, the slides were fixed in absolute ethanol at 4ºC for 10 min, dried, and stored at -70ºC.
IIF procedure. The thawed slides were washed with phosphate-buffered saline (PBS), pH 7.5, and 25 µl of the test serum was added to each well at serial dilutions from 1:16 to 1:512. The slides were incubated at 37ºC for 30 min in a humid chamber. Each batch of test sera was accompanied by a 1:16 dilution of positive (C and P standards) and negative controls, and PBS. After washing with PBS, the slides were incubated in duplicate for 30 min with fluorescein conjugated sheep anti-human IgG or IgM (Dako, Copenhagen, Denmark), washed, and mounted for reading.
The slides were read by two independent examiners who were not aware of each other's results. All fluorescence present in the cytoplasm or nucleus of the cells and not compatible with the C or P standard was considered to be an atypical pattern (A-ANCA).
Detection of antinuclear antibodies (ANA)
Testing for ANA was done to exclude false-positive ANCA results. Slides with 3-µm thick normal mouse liver sections were incubated with a 1:16 dilution of serum and later with fluorescein conjugated sheep anti-human IgG or IgM (Dako), washed, and mounted for reading. The result was considered positive when there was fluorescence in the nuclear region of the hepatocytes (13).
Detection of ANCA by ELISA
In patients with leproniatous leprosy, an antigen capture ELISA was used as previously described (') to test sera for the presence of PR3, MPO, or HLE antibodies. Monoclonal antibodies directed against PR3 [12.8; Central Laboratory of the Netherlands Red Cross Blood Transfusion Service (CLB), Amsterdam, The Netherlands], MPO (14.15: CLB), and HLE (M752; Dakopatts. Copenhagen, Denmark) were used.
Statistical analysis
The results are presented as the means ± S.D. Comparisons between proportions were made by the normal distribution at 5% level of significance (p < 0.05). Comparisons between sexes, ages, clinical forms of leprosy, reactional states, disease activity, disease durations, therapeutic regimens, and ANCA positivity were done by Fisher's exact test: then the level of significance used was less than 0.01 (9).
RESULTS
The control subjects had a mean age of 26.9 ± 5.6 years (range 19-38); 42 were males and 18 females. Neither ANCA nor ANA were detected in their sera with antihuman IgG or with IgM conjugate.
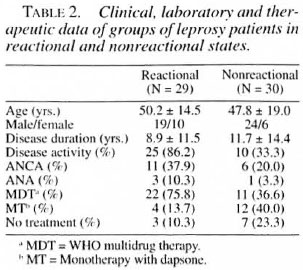
The leprosy patients had a mean age of 48.9 ± 16.8 years (range 8-74); 43 were males and 16 females. The mean disease duration was 10.3 ± 13.0 years (range 0.001-53); 29 were reactional and 30 nonreactional, and 35 (59.3%) presented disease activity. The therapy for leprosy was multidrug therapy recommended by the World Health Organization (WHO/MDT)(34,35) in 33 (55.9%) patients or monotherapy with dapsone (MT) in 16 (27.1%); 10 patients (19.9%) were not under treatment. By IIF with anti-human IgG conjugate, ANCA were present in 17 (28.8%) sera, all of them showing an atypical pattern (A-ANCA). No serum tested showed positive fluorescence in the IIF test carried out with anti-human IgM conjugate. ANA was detected in 4 (6.7%) sera of leprosy patients. By ELISA, anti-PR3, MPO, or HLE antibodies were not present in the sera of lepromatous leprosy patients. The clinical, laboratory and therapeutic data for patients with different forms of leprosy and for the reactional and nonreactional groups are presented, respectively, in Tables 1 and 2.
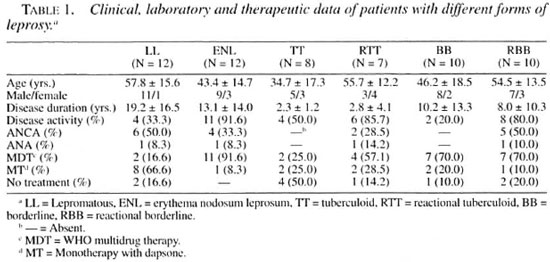
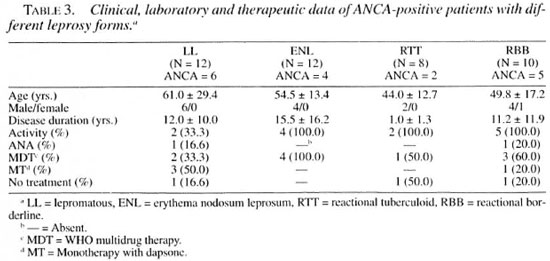
The mean age of the 17 ANCA-positive leprosy patients was 54.1 ± 16.4 years (range 22-73); 16 were males and 1 female; the mean disease duration was 11.2 ± 11.9 years (range 0.08-36), 11 were reactional and 6 nonreactional, and 13 (76.4%) presented disease activity. The treatment was WHO/MDT in 10 (58.8%) patients and MT in 4 (23.5%); 3 patients (17.6%) were not under treatment. The clinical, laboratory and therapeutic data for ANCA-positive patients with different forms of leprosy are shown in Table 3. The 42 (71.1%) ANCA-negative leprosy patients had a mean age of 48.3 ± 16.6 years (range 8-74), 27 were males and 15 females; the mean disease duration was 9.2 ± 13.1 years (range 0.04-53), 13 were reactional and 24 nonreactional, and 22 (52.3%) presented disease activity. The treatment was WHO/MDT in 23 (54.7%) patients and MT in 12 (28.5%); 7 (16.6%) patients were not under treatment.
The analysis did not show statistical significance (p > 0.05) between the presence of ANCA and either ages, clinical forms of leprosy, reactional states, disease activity, duration of disease, or therapeutic regimen. The p values were statistically significant (p < 0.01) between ANCA positivity in leprosy patients and in control subjects, and presence of ANCA and gender (males).
DISCUSSION
The hypothesis of this study was that ANCA may be involved in leprosy as a marker of the specific type linked to the presence of vasculitis. The results show that atypical ANCA (A-ANCA) are present in 28.8% of leprosy patients. However, there was no correlation between ANCA positivity and either clinical forms of leprosy, reactional state, disease activity, or disease duration (p > 0.05).
Despite the fact that ANCA have been described, with variable frequency, in many infectious diseases (14, 22, 28, 30, 32)and that their active participation in the pathogenesis of systemic and/or cutaneous autoimmune vasculitis are increasingly more evident (16 ,17, 20), the presence of these autoantibodies in the different forms of leprosy has not been extensively studied.
The first report about ANCA and leprosy was published in 1995 by Freire, et al . (12), who observed the absence of ANCA in the serum of 10 patients with ENL whose lesions were confirmed by bacilloscopy and by histologic evidence of vasculitis. Recently, Medina, et al. (23) have described ANCA in 17.1% of a series of 64 leprosy patients (9 P-ANCA and 2 C-ANCA), mainly of the lepromatous pole, and did not find any correlation between ANCA and the reactional state or ANCA and disease activity.
In our study, none of the 60 normal subjects showed the presence of ANCA. To the best of our knowledge ANCA have not been detected in healthy populations, being present only under pathological conditions. Nevertheless, it is still unclear whether ANCA play a pathogenic role or are only an epiphenomenon in most of the ANCA-related diseases (20). Our finding of A-ANCA positivity in 28.8% of leprosy patients without correlation with clinical forms of the disease suggests that ANCA do not play a pathogenic role in the disease, being an epiphenomenon similar to the other organand nonorgan-specific autoantibodies reported in leprosy such as ANA, cryoglobulins, anticardiolipins, anticytoskeletal, antispermatozoal, antithyroglobulin, antigastric mucosa antibodies, etc. (2-5, 15, 18, 31).
Autoantibodies have been reported predominantly in the lepromatous form of leprosy, which is a multibacillary form with marked humoral immune response. In the tuberculoid form, which is paucibacillary and presents marked cellular immune response, the presence of autoantibodies is less frequent (3-5, 15, 18, 24, 26, 31) . In the present study, the ANCA positivity was higher, although not significantly so (p > 0.05), in lepromatous patients (41.6% versus 20.0%) and in the reactional group (37.9% versus 20.0%). These results reenforce the hypothesis that ANCA in leprosy are an epiphenomenon, presumably resulting from polyclonal B-cell activation.
Contrary to what was expected, the vasculitis of the ENL seems not to be ANCA-related, since these autoantibodies were found less frequently, although not significantly (p > 0.05), in patients with ENL than in patients with the nonreactional lepromatous form (33.3% versus 50.0%). These findings, in accordance with those by Freire, et al. (12), might be indirect evidence that the pathogenesis of the ENL vasculitis involves a Mycobacterium leprae -specific humoral response with local immune complex deposition and complement fixation, as has been reported (3-5, 24-26).
The origin of some autoantibodies in leprosy has been explained, although not definitively, by crossreactions. Thus, ANA would result from crossreactivity with complex nucleic acids and nucleoproteins, and anticytoskeletal antibodies from crossreactivity between mycobacterial proteins and human intermediate filament protein subunits (15). Heat-shock proteins (HSP) are a phylogenetically conserved family of proteins, induced during chronic inflammation and other forms of physiological stress, that lead to both humoral and cellular immune responses against microorganisms(8)). Therefore, HSP may stimulate autoimmune phenomena in vivo during chronic mycobacterial infection. It has been demonstrated that HPS65 of M. leprae is recognized with specificity by antibodies against human LF (one of the ANCA antigens) and that bacterial debris keep the antigenicity for anti-LF antibodies even after the death of the mycobacteria (8). In the ELISA performed on the lepromatous patients' sera we did not address this question, but further investigations should contemplate the possibility of reactivity to HSP being implied in the genesis of ANCA in leprosy.
Since it has been shown that certain drugs, such as minocycline (7) and propylthiouracil (11), can induce ANCA, we also tested the possibility that drugs used for leprosy treatment could cause production of these autoantibodies. However, our results did not show any correlation (p > 0.05) between ANCA and therapeutic regimens, suggesting that ANCA is not drug-induced in leprosy.
In all positive sera, the IIF pattern was atypical, i.e., A-ANCA. The antigens involved in this type of ANCA pattern are unknown. Thus far, no correlation has been established between A-ANCA and any clinical entity, and some authors consider A-ANCA to be nonspecific (16). A-ANCA can be detected in ANA-positive sera and at times, after further dilution, the A pattern turns out to be a P pattern. In the present study, only two ANA-positive sera exhibited an A pattern and the type of fluorescence did not change at higher dilutions. ELISA carried out on the sera of lepromatous leprosy patients did not show activity against PR-3, MPO, or HLE. Thus, the ANCA detected in patients with the lepromatous form of leprosy are not active against the best known ANCA antigens. The report by Medina, et al. (23) of C- and P-ANCA, whose major antigens are PR-3 and MPO, respectively, also suggests that in leprosy ANCA can be directed against different enzymes. The C-ANCA without central accentuation (atypical) detected in chromomycosis did not react with PR-3, showing that in other granulomatous chronic infectious disease ANCA is not directed against the most frequent ANCA antigens (14).
Another interesting finding of the present report is the association between ANCA and gender: 94.1% of ANCA-positive patients were male (p < 0.01). So far ANCA has not been related with gender and further investigations are necessary to determine whether there is a gender influence on ANCA production in leprosy.
In conclusion, the results of this work suggest that ANCA are not related to the presence of vasculitis in the reactive forms of leprosy of the ENL type and do not represent a marker of a specific clinical form of the disease. The hypothesis of the present study, which was based on the similarities between leprosy and WG, was recently supported by the description of a case of borderline lepromatous leprosy whose autopsy revealed, in addition to the extensive specific involvement of leprosy, pulmonary granulomatous necrotizing vasculitis with no evidence of bacilli or fungi, compatible with WG (10). This may have been a random association, but there is the possibility that the infectious process may trigger autoimmune phenomena inducing necrotizing vasculitis. Since leprosy does not involve the lungs (1), more in-depth investigation is needed for leprosy patients with pulmonary symptoms and/or radiologic alterations for a WG diagnosis.
Acknowledgment. The authors are grateful to Dr. CeesG. M. Kallenberg. Department of Rheumatology and Clinical Immunology, University of Groningen, for his generous help; to Dr. Lídia Raquel de Carvalho. Department of Biostatistics. Botucatu Institute of Biosciences, for the statistical analysis, and to Drs. Adrian O. Vladutiu. Department of Pathology, State University of New York at Buffalo, and Álvaro Oscar Campana, Department of Internal Medicine, Botucatu Medical School, for their useful discussions. This work was supported by a grant (95/1545-0) from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP).
REFERENCES
1. ÁGUAS, J. T. Lepra visceral y endocrina. Rev. Leprol. Fontilles 20(1996)1101-1114.
2. BRÁS. A. and ÁGUAS, A. P. Mycobacteria-induced autoantibody production is associated with susceptibility to infection but not with host propensity to develop autoimmune disease. Clin. Exp. Immunol. 100(1995)75-80.
3. BUCHANAN. T. M. Serology of leprosy. In: Leprosy. Hastings, R. C. ed. Edinburgh: Churchill Livingstone. 1994, pp. 169-176.
4. CHÁVEZ-LEGAZPI, M., GÓMEZ-VÁSQUEZ, A. and GARCIA-DE LA TORRE, I. Study of rheumatic manifestations and serologic abnormalities in patients with lepromatous leprosy. J. Rheumatol. 12(1985)737-741.
5. CHOUDHURI, K. The immunology of leprosy; unraveling an enigma. Int. J. Lepr. 63(1995)430-447.
6. COHEN TERVAERT, J. W., GOLDSCHMEDING, R., ELEMA. J. D., VAN DER GIESSEN, M.. HUITERNA, M. G, VAN DER HENM, G. K., THE, T. H., VON DEM BORNE, A. E. G. KR. and KALLENBERG, C. G. M. Autoantibodies against myeloid lysosomal enzymes in crescentic glomerulonephritis. Kidney Int. 37(1990)795-806.
7. ELKAYAM, O., YARON, M. and CASPI, D. Minocycline induced arthritis associated with fever, livedo reticularis, and pANCA. Ann. Rheum. Dis. 55(1996)769-771.
8. ESAGUY, N., ÁGUAS, A. P., VAN EMBDEN, J. D. A. and SILVA. M. T. Mycobacteria and human autoimmune disease: direct evidence of cross-reactivity between human lactoferrin and 65-kilodalton protein of tubercle and leprosy bacilli. Infect. Immun. 59(1991)1117-1125.
9. FISHER, L. D. and VAN BELLE, G. Biostatistics: A Methodology for the Healthy Sciences. New York: John Wiley & Sons. 1993.
10. FLEURY, R. N.. TABORDA, P. R. O. and OPROMOLLA, D. V. A. Granulomatose de Wegener e hanseníase: apenas uma associação fortuita? Hansenol. Int. 21(1996)43-53.
11. FREIRE, B. F. A., KOBAYASHI, A. S.. PAULA, I. D. and QUELUZ. T. T. Does prolonged treatment with propylthiouracil (PTU) induce ANCA? Clin. Exp. Immunol. 101 S-l (1995)68.
12. FREIRE, B. F. A. . NAKAYAMA, E. E. and QUELUZ, T. T. Absences of anti-neutrophil cytoplasmic antibodies (ANCA) in erythema nodosum as a leprosy reactions. Clin. Exp. Immunol 101 S-l (1995)69.
13. FRITZLER, M. J. and TAN, E. M. Antinuclear antibodies and the connective tissue diseases. In: Laboratory Diagnostic Procedures in the Rheumatic Diseases. 3rd edn. Cohen, A. S.. ed. Orlando: Grune and Stratton. Inc., 1985. pp. 207-248.
14. GALPERIN, C. SHOENFELD, Y, GILBURD, B., ESTERRE, P., MERONI, P. L.. HALPERN, G. M.. ANDRIANTSIMAHAVANDY, A. and GERSHWIN, M. E. Antineutrophil cytoplasmic antibodies in patients with chromomycosis. Clin. Exp. Rheumatol. 14(1996)479-483.
15. GARCIA-DE LA TORRE, I. Autoimmune phenomena in leprosy, particularly antinuclear antibodies and rheumatoid factor. J. Rheumatol. 20(1993)900-903.
16. GROSS, W. L., CSERNOK, E. and HELMCHEN, U. Antineutrophil cytoplasmic autoantibodies, autoantigens. and systemic vasculitis. APMIS 103(1995)81-97.
17. GROSS, W. L., HANSCHILD, S. and MISTRY, N. The clinical relevance of ANCA in vasculitis. Clin. Exp. Immunol. 93 S-l (1993) 7-11.
18. GUEDES BARBOSA, L. S., GILBRUT, B., SHOENFELD, Y. and SCHEINBERG, M. A. Autoantibodies in leprosy sera. Clin. Rheumatol. 15(1996)26-28.
19. INTERNATIONAL CONGRESS OF LEPROSY. Report of Committee on classification, Madrid. 1953. Int. J. Lepr. 21(1953)504-516.
20. KALLENBERG, C. G. M.. BROUWER, E., WEENING, J. J. and COHEN TERVAERT, J. W. Anti-neutrophil cytoplasmic antibodies: current diagnostic and pathophysiological potential. Kidney Int. 46(1994)1-15.
21. KALLENBERG. C. G. M., MULDER. A. H. L. and COHEN TERVAERT, J. W. Antineutrophil cytoplasmic antibodies: a still-growing class of autoantibodies in inflammatory disorders. Am. J. Med. 93(1992)675-682.
22. KLAASSEN , R. J., GOLDSCHMEDING, R., DOLMAN, K. M., VLEKKE., A. B., WEIGEL, H. M., EEFTINCK-SCGATTENKERK, J. K., MULDER, J. W., WESTEDT, M. L. and VON DEM BORNE, A. E. Anti-neutrophil cytoplasmic autoantibodies in patients with symptomatic HIV infection. Clin. Exp. Immunol. 87(1992)24-30.
23. MEDINA, F., CAMARGO, A., MORENO, J., ZONANA NACACH, A., ACEVES AVILA, J. and FRAGA, A. Anti-neutrophil cytoplasmic autoantibodies in leprosy. Br. J. Rheumatol. 37(1998)270-273.
24. NAAFS, B. Leprosy reactions. Trop. Geogr. Med. 46(1994) 80-84.
25. NUNDY, S. Leprosy: report of a meeting of physicians and scientists at the All India Institute of Medical Sciences, New Delhi. Lancet 345(1995)697-703.
26. OTTENHOFF, T. H. M. Immunology of leprosy. Trop. Geogr. Med. 46(1994)72-80.
27. PINCHING, C. A., REES. A. J., PUSSELL, B. A., LOCKWOOD, C. M., MITCHINSON, R. S. and PETERS, D. K. Relapses of Wegener's granulomatosis: the role of infection. Br. Med. J. 28(1980)836-838.
28. PUFIDIN, D. J., DUURSMA, J.. GARHIRAN, V. and JACKSON. T. F. Invasive amoebiasis is associated with the development of anti-neutrophil cytoplasmic antibodies. Clin. Exp. Immunol. 12(1994)48-51.
29. RASMUSSEN, N. and WIIK, A. Indirect immunofluorescence examination for IgG-ANCA in sera submitted for the First International Workshop on ANCA. APMIS 97 S-6 (1989)16-20.
30. SCHMITT, W. H., CSERNOCK, E, and GROSS, W. L. ANCA and infection. Lancet 337(1991)1416-1417.
31. SHARMA, V. K.. SAHA, K. and SEHGAL, V. N. Serum immunoglobulins and autoantibodies during and after erythema nodosum leprosum. Int. J. Lepr. 50(1982)159-163.
32. SOTO, A., JORGENSEN, C, OKSMAN. F, NOIL, L. H. and SANY, J. Endocarditis associated with ANCA. Clin. Exp. Rheumatol. 12(1994)203-204.
33. VAN DER WOUDE, F. J., RASMUSSEN, N., LOBATTO, S., WIIK, A., PERMIN, H., VAN ES, L. A., VAN DER GIESSEN, M., VAN DER HEM, G. K. and THE, T. H. Autoantibodies against neutrophils and monocytes: tool for diagnosis and marker of disease activity in Wegener's granulomatosis. Lancet 23(1985)425-429.
34. WHO EXPERT COMMITTEE ON LEPROSY. Sixth report. Geneva: World Health Organization, 1988. Tech. Rep. Ser. 768.
35. WHO STUDY GROUP. Chemotherapy of leprosy for control programmes. Geneva: World Health Organization. 1982. Tech. Rep. Ser. 675.
36. WIIK, A. Delineation of a standard procedure for indirect immunofluorescence detection of ANCA. APMIS 97 S-6 (1989)12-13.
37. WIIK, A., STUMANN, L., KJELDSEN, L., BORRE-GAARD, N., ULLMAN, S., JACOBSEN, S. and HALBERG, P. The diversity of perinuclear antineutrophil cytoplasmic antibodies (pANCA) antigens. Clin. Exp. Immunol. 101 S-l (1995) 15-17.
1. M.D., Ph.D.
2. M.D.
3. M.D.
4. M.D., Lauro de Souza Lima Institute, Bauru. SP. Brazil.
5. M.D.. Ph.D., Department of Internal Medicine. Botucatu Medical School. Universidade Estadual Paulista (UNESP). Botucatu, SP 18618-000. Brazil.
Reprint requests to Dr. Queluz, at the above address or e-mail: queluz@laser.com.br
Received for publication on 18 May 1998.
Accepted for publication in revised form on 14 October 1998.