- Volume 65 , Number 1
- Page: 28–36
A study on the effectiveness and safety of the WHO/MDT regimen in the northeast of thailand; a prospective study, 1984-1996
ABSTRACT
The aim of this prospective study was to determine the effectiveness and safety of the multidrug therapy as recommended by the World Health Organization (WHO/ MDT) in 1982. One-hundred-eighty-eight newly diagnosed leprosy patients 1130 paucibacillary (PB) and 58 multibacillary (MB) patients] f rom three provinces in northeastern Thailand were recruited into a study f rom April 1984 to March 1985. The study lasted until May 1996. The results showed that 182 patients finished their course of WHO/MDT, representing a treatment completion rate of 95%; 167 (122 PB and 45 MB) were released f rom surveillance (RES); 82 PB patients were still available for follow up by the end of 1994 and 31 MB patients by May 1996. Two PB patients were diagnosed with a relapse, showing a relapse rate of 0.2 per 100 person-years at risk. After an average of 8 years of follow up, no MB relapses have been diagnosed. The proportion of patients with a WHO grade 2 disability among PB and MB patients increased f rom 4% and 8% at the start of treatment to 7% and 13% at last examination, respectively. It is concluded that the fixed-duration, 6 month WHO/MDT regimen tor PB leprosy and the 24-month regimen for MB leprosy are effective, acceptable and safe, and that clinical activity, histopathological activity and/or a positive skin smear at release f rom treatment (RFT) have no bearing on the efficacy of the WHO/MDT regimens. The relapse rates are low and in accordance with most published data available to date. The importance of skin-smear services for a reliable classification (WHO PB/MB classification for control programs) is stressed.RÉSUMÉ
Le but de cette étude en perspective était de déterminer l'efficacité et l'absence de danger de la polychimiothérapie telle que recommandée par l'Organisation Mondiale de la Santé (PCT/OMS) en 1982. Cent quatre-vingt-huit nouveaux malades de la lèpre [130 paucibacillaires (PB) et 58 multibacillaires (MB )] provenant de trois provinces dans le nord-est de la Thaïlande ont été recrutés entre avril 1984 et mars 1985 pour une étude. Cette étude dura jusqu'en mai 1996. Les résultats ont montré que 182 malades ont terminé leur traitement de PCT/OMS, ce qui fait un taux de completion de 95%; 167 ( 122 PB et 45 MB) ont été libérés des contrôles (LDC); 82 patients PB pouvaient encore être suivis en fin de 1994, et 31 MB en mai 1996. Deux rechutes ont été diagnostiquées chez des patients PB, montrant un taux de rechute de 0.2 pour 100 PYR. Après une moyenne de 8 années de suivi, aucune rechute MB n'a été diagnostiquée. La proportion de patients avec une incapacité de grade 2 parmi les PB et les MB a augmenté, passant respectivement de 4% et 8% au début du traitement, à 7% et 13% au dernier examen. On en conclut que les régimes de PCT/OMS de durée fixe de 6 mois pour la lèpre PB et de 24 mois pour la lèpre MB sont efficaces, acceptables et sans danger, et que l'activité clinique, l'activité histopathologique et/ou la positivité de frottis cutanés à l'arrêt du traitement n'influencent pas l 'efficacité des régimes de PCT/OMS. Les taux de rechute sont bas et en accord avec la majorité des données disponibles publiées à ce jour. L'importance des services de frottis cutanés pour une classification fiable (classification OMS PB/MB pour les programmes de contrôle) est soulignée.RESUMEN
El objeto del estudio eu perspective fue el determinar la efectividad y seguridad de la poliquimioterapia (PQT) recomendada por la Organización Mundial de la Salud en 1982 (PQT/OMS). Se estudiaron 188 casos nuevos de lepra (130 paucibacilares, PB, y 58 multibacilares, MB) provinientes de 3 provincias del noreste de Tailandia, reclinados entre abril de 1984 a mar/o de 1985. El estudio duró hasta mayo de 1996. Ciento ochenta y dos pacientes (95% del total) terminaron el tratamiento de PQT/OMS; 167 casos (122 PB y 45 MM) fueron dados de alta al término del tratamiento; 82 pacientes Pl! pudieron ser explorados todavía a finales de 1994 y 31 pacientes MB en mayo de 1996. En dos pacientes se diagnosticaron recaídas, lo que representó una tasa de recaída de 0.2 por 100 PYR. En los cases MB no se registraron recaídas después de un tiempo promedio de seguimiento de 8 años. En el último examen se encontró que la proporción de pacientes con disfunción de grado 2 (OMS), aumentó del 4% (en los casos PB) y del 8% (en los casos MB) al inicio del tratamiento, al 7% en los casos PB y al 1.3% en los casos MB. Se concluye que la duración de la PQT lijada por la OMS, de 6 meses para la lepra PB y tic 24 meses para la lepra MB, es efectiva, aceptable y segura. Las tasas de recaída son bajas y en corcondancia con la mayoría de los datos publicados a la fecha. Se subraya la importancia de los extendidos de linfa cutánea para lograr una clasilicaión confiable (WHO PB/MB) por programas dirección.In 1981 the World Health Organization (WHO) Study Group on the Chemotherapy of Leprosy for Control Programmes recommended multidrug regimens (WHO/MDT) for both multibacillary (MB) and paucibacillary (PB) patients (18). At that time there was insufficient scientific evidence to support the efficacy and safety of WHO/MDT. On the request of the Ethics Committee of the Thai Ministry of Public Health, a pilot study was undertaken to study the effectiveness, acceptability and safety of the WHO recommended MDT regimens and to measure the occurrence of complications and relapses. This paper reports on the experience with WHO/MDT for PB and MB patients in the leprosy control programs of three provinces in northeastern Thailand. A comparison is made between clinical/bacteriological (by skin smear) classification and the reliability of clinical classification based on numbers of skin lesions and body areas involved. Findings on clinical and histopathological activity, skin-smear positivity at release from treatment (RFT) and its resolution, and skin-smear negativity are presented. The occurrence of relapses and late reactions and the difficulties in differentiating between relapses and late reactions are discussed. The proportions of patients with WHO disability grade 2 at the start of treatment and at last examination are compared.
MATERIALS AND METHODS
Only new cases of leprosy with no prior treatment were included in this prospective study from April 1984-March 1985. The area chosen for the pilot study covered three provinces (Mahasarakham, Kalasin and Roi-et) in northeastern Thailand, where a vertically organized leprosy control program is in operation (12). In 1984 the area had a population of 2,740,000 with a known leprosy prevalence rate of 28/10,000. Approximately 40% of the new cases during that period agreed to participate in the study.
All cases were examined, classified and followed up by the research team, consisting of staff from the Regional Leprosy Control Centre of the northeast and from the Leprosy Division, Ministry of Public Health, Bangkok. The patients were seen by the research team at the three provincial leprosy control offices every 6 months until 3 years after RFT. After that the patients were followed up by the regional LCC team, but less regularly. In 1994/1996 the study patients were traced and re-examined.
While on treatment, the patients were seen monthly by their assigned health workers and given the supervised dose of WHO/MDT. Neither the urine spot test for the presence of dapsone nor tablet counts at patients' homes was undertaken. A specially designed form with a list of side effects of the WHO/MDT drugs had to be filled in during the monthly visits. While on treatment and at RFT patients were given health education about the signs and symptoms of the side effects of drugs, reactions, neuritis and relapse. Patients were instructed to report to the health worker immediately if any such signs or symptoms were noticed.
Skin smears and biopsies were taken to confirm the diagnosis and classification and to monitor the patients during treatment and surveillance. The premise was that the clinical/bacteriological (by skin smear) classification would be the "gold standard"; the histopathology according to Ridley-Jopling (17) was used to support the clinical/bacteriological classification. Indeterminate cases (with negative skin smears) were not recruited into the study because of the uncertainty surrounding clinical and histopathological stages, which could cloud the evaluation of the effectiveness of the treatment. After RFT, if the biopsy still showed active signs of inflammation, it would be repeated annually. In all cases clinical lesions were photographed before and after treatment.
PB leprosy included the clinically diagnosed polar tuberculoid (TT) and borderline tuberculoid (BT) leprosy with a bacterial index (BI) of < 2+ at all sites. MB leprosy included the clinically diagnosed polar lepromatous (LL), borderline lepromatous (BL), and borderline (BB) leprosy, as well as any other types with a BI of 2+ or more at any site. The patients were given the WHO/MDT regimen (6 doses in 9 months for PB leprosy and 24 doses within 36 months for MB leprosy). After completing the prescribed fixed-duration regimen, patients were RFT even if the skin smears were still positive. PB patients were released from surveillance (RFS) 3 years and MB patients 5 years after RFT. In the event of any complications, the patients were advised to report to the provincial leprosy control office.
Criteria to determine clinical inactivity were: 1) patches and nodules were neither raised nor red, or had disappeared altogether; 2) the number and size of skin lesions did not increase for the last half year; 3) nerves were not recently enlarged and were not tender on palpation. Criteria to determine histopathological activity were the presence of epithelioid cell granuloma in the tuberculoid type and macrophage granuloma in the lepromatous type. The biopsy slides were examined independently by two dermatologists who were in agreement on the final diagnosis.
Late reversal reaction (late RR) was categorized into mild and severe reaction. Mild reaction was defined as the reappearance of old lesions which had completely disappeared, or remaining lesions becoming more clearly visible or erythematous while severe reaction involved previous lesions and/or nerves as defined by Rose and Waters (13). The definition of a late erythema nodosum leprosum (ENL) reaction was the appearance after RFT of mild or severe ENL, without an increase in the BI of the skin smear. A late reaction should be differentiated from a relapse. A relapse was defined as the appearance after RFT of new lesions or the increase in size of an old lesion; a skin smear becoming positive in a previously PB patient or an increase in the BI at any site of more than 1+ in a previously skin-smear-positive patient (2).
The previously used 4-grade WHO disability classification was transferred to the new 3 - grade classification (l7). In 1984/1985 only WHO disability grades were recorded. Since 1987, with the introduction of a prevention of disability (POD) form, sensory and voluntary muscle tests have been recorded by the control program.
RESULTS
A total of 188 newly registered patients were recruited into the study: 130 (69%) were PB and 58 (31%), MB; 61% were males and 39% females; 12% among them were children. The mode of detection was as follows: 67% by voluntary report, 19% by rapid village survey, and 13% by contact examination. No patient was changed from PB to MB on the basis of the histopathological or bacteriological findings.
Clinical classification-number of skin lesions/number of body areas involved. We looked at the clinical classification solely on the basis of the number of skin lesions without taking into consideration the number of nerve lesions involved. Patients with up to five lesions were assigned to the PB regimen and those with six or more to the MB regimen. The outcome was compared with the result of the clinical-bacteriological classification and showed that 12% of the true MB patients would be undertreated (MB patients receiving PB regimen) while 12% of the true PB patients would be overtreated (Table 1).
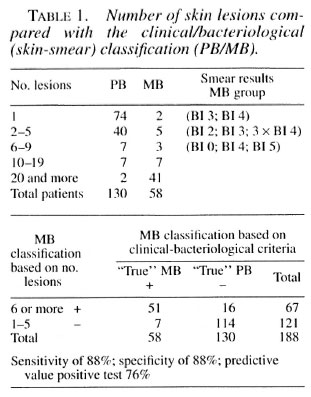
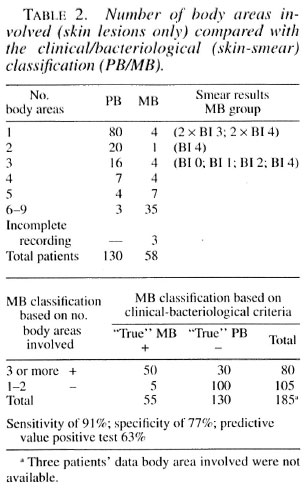
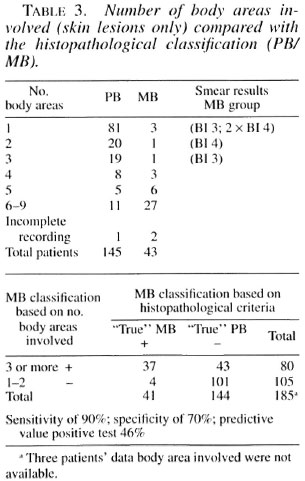
If we considered the clinical classification based only on skin lesions and body area involved (l4), omitting nerve lesions and without bacteriological confirmation, patients with up to two body areas affected were assigned to the PB regimen and those with three or more areas to the MB regimen. When the outcome was compared with the results of the clinical-bacteriological classification, 9% of the true MB patients with up to two body areas involved would be undertreated and 23% of the true PB patients with three or more body areas involved would be ovcrtreated (Table 2). If the outcome (body area count) was compared with the results of the histopathological classification, 10% of the MB patients with up to two body areas involved would be undertreated and 30% of the PB patients with three or more body areas involved would be overtreated (Table 3).
RFT. A total of 182 patients, 129 PB and 53 MB, were released from treatment: 179 patients (127 PB and 52 MB) finished WHO/MDT within the prescribed period, representing a completion (TC) rate of 95% and the remaining 3 patients (2 PB and 1 MB) finished at a later date. Two (1 BL, 1 LL) out of 52 MB patients who suffered from chronic ENL received WHO/MDT MB for a total of 36 and 48 months, respectively.
Skin-smear results. At RFT 26 patients were found to be skin-smear positive: 3 PB (BT) patients previously skin-smear negative were found to have a BI of 1+ at one site only; 15 out of 34 BL and all 8 LL cases were still skin-smear positive. The majority of patients with positive smears at RFT became negative within 3 years after-ward. Only two patients took more than 4 years to become negative.
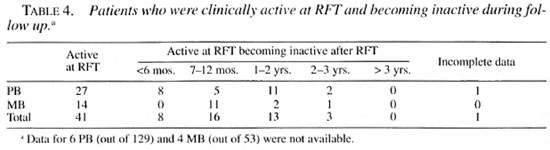
Clinical activity/inactivity. Twenty-seven out of 123 PB (22%) and 14 out of 49 MB (29%) were considered still clinically active at RFT; 8% TT patients, 27% BT, 0% BB, 23% BL and 88% LL patients. The length of time from clinical inactivity is shown in Table 4.
Histopathology. Histopathological examination showed that 57 out of 121 PB (47%) and 17 out of 48 MB (35%) were still histopathologically active after RFT; 27% TT patients, 56% BT, 4% BB, 32% BL and 75% LL. Table 5 shows the length of time from histopathological activity to inactivity; patients who were inactive at RFT but later on developed a reaction and became histopathologically active again have not been shown.

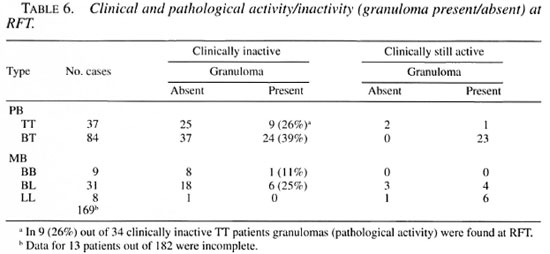
In many PB patients considered clinically inactive at RFT (26% TT and 39% BT), histopathological activity was still found. All of the 23 BT patients considered clinically active at RFT had granuloma present on examination. This was found to a lesser extent in the MB group, 25% of the 24 BL patients (Table 6). Most clinically active patients became inactive within 2 years after RFT (Table 5).
Surveillance and follow up after RFS. One-hundred-seventy-seven patients (124 PB and 53 MB) were followed up after RFT and 167 (122 PB and 45 MB) were actually released from surveillance (RFS). Eighty-two PB patients were still available for follow up by the end of 1994 and 31 MB patients were followed up until May1996.
Late reactions. Twelve out of 124 PB patients (10%) suffered from a late reaction (8% mild late RR and 2% severe late RR) and 7 out of 53 MB patients (13%) suffered from a later reaction (6% mild late RR, 6% severe late RR and 2% mild ENL reactions) in the fust 4 years after RFT.
Relapses among PB group. Two relapses were diagnosed in the follow-up group of 112 patients: 1 BT patient developed new lesions and suffered a flare up of old lesions 5.5 years after RFT but remained skin-smear negative; another BT patient with a Bl of 1+ at the start of treatment developed a relapse with a positive skin smear (BI of 4+) 7.5 years after RFT. Those 1 12 patients were followed up after RFT from I to 10 years (mean 8.2 years). The total number of person-years of follow up of PB patients was 921. The PB relapse rate was 0.2 per 100 person-years of follow up.
Relapses among MB group. None of the MB patients developed signs of relapse. Fifty-one MB patients were followed up after RFT for 2 to 10 years (mean 8 years). The total number of person-years of MB patients follow up was 416. At the start of treatment 24 MB patients had a Bl of 4+ (highest Bl of any site, not the average BI) and 12 patients a BI of 5+ or more. Fifteen out of the 24 with a BI of 4+ and 6 out of the 12 with a BI of 5+ were followed up until May 1996 and none was found to have relapsed.
Impairments. At the start of the study in 1984/1985, only the WHO disability grading was done. Therefore, we only had that to compare with our findings at the last examination (1992-1996). The proportion of PB patients with disability grade 2 increased from 4% to 7%; that of MB patients, from 8% to 13%.
Side effects. In this group of 188 patients one developed rifampin hepatitis. No other side effects of rifampin, such as the flu syndrome or shock, were found. No cases of dapsone allergy were encountered.
DISCUSSION
The relapse rate provides the ultimate proof of the successful treatment of infectious diseases, even though regimens also need to be assessed in terms of acceptability, incidence of toxic side effects, duration and ease of treatment, and cost (15). The outcome of case management regarding new/additional impairments was not looked into specifically in this study. In general, we can say that the MDT regimens as recommended by WHO in 1982 were effective, that there was no problem with compliance among the patients, and that the incidence of adverse drug effects was low.
Outcome of fixed WHO/MDT regimen-relapses. The outcome (chemotherapy) of both the fixed duration PB and MB regimens was very satisfactory: 2 relapses were seen after more than 900 person-years of follow up in the PB group (from 1985-1994) and none in the MB group after 416 person-years of follow up (1986/1987-1996). These results compare well with other studies on relapse in leprosy (15,20). The patient who had a BI of 1+ at the start of treatment and who developed a relapse after WHO/MDT PB would today be assigned to the MB group from the start (20). We cannot confirm the findings of the Marchoux Chemotherapy Study Group that show a high relapse rate after long-term follow up of MB patients treated with the WHO/MDT regimen (11,16).
Fixed regimen and late reactions. In a few cases the study protocol (fixed treatment regimen of six doses in the PB group) was not strictly adhered to. For example, some PB patients were given a new course of WHO/MDT when signs of a late reaction developed, especially when the pathologist reported granuloma in the skin biopsy and suggested a possible relapse. The finding of granuloma in the skin lesions during the first year after stopping MDT appears to be no proof of a relapse and can be observed in a relapse as well as in a late reaction (13). None of the patients with a late reaction who did not receive additional courses of WHO/MDT developed a relapse.
Diagnosis and classification. The classification in PB and MB was made on clinical and bacteriological grounds as advised by the WHO in 1982. This classification was considered in our study to be the "gold standard." However, we are aware that in some clinically diagnosed PB cases, with negative skin smears, bacilli can be found in the nerves by histopathology. Both of the BT patients in our study who relapsed had only two lesions in only two body areas. It is suggested that skin-smear-negative BT patients with more extensive lesions are more likely to have a positive BI in the nerve biopsy (l4). In a PB patient with a negative skin smear a BI of 1+ in the nerve should be considered just by chance. However, if in such a patient many bacilli were found in the nerve (BI 2+ or more) should that patient be considered an MB case (l7)? There was one BL patient in our study with a repeatedly negative skin smear, although clinically and histopathologically he was a BL case. The finding of negative skin smears in BL patients seems to be not uncommon (6). In 1986 Thailand and in 1988 WHO changed the classification to the extent that clinical BT patients with a positive BI at any site were to be treated as MB (16).
Reliable skin-smear examinations may contribute to the reliability of the classification which, in turn, will have a bearing on the PB relapse rate (8). In view of the experience that skin-smear-negative BT patients (PB leprosy) with extensive disease are more likely to relapse, together with the difficulties in obtaining reliable smear results in the field (14,17), today more reliance is placed on clinical examination alone for the classification of leprosy. The characteristics of skin and nerve lesions are important in the differentiation between PB and MB leprosy (4,10). However, it requires much clinical experience to classify the majority of patients, in particular the BT group. Instead, it has been proposed to use the number of skin lesions or the number of body areas with skin/nerve lesions (l7) to derive a clinical classification. Classification criteria should be aimed at correctly classifying a maximum number of MB cases (this is the sensitivity of the criteria), while limiting the number of cases wrongly classified as MB (the "false positives" among those classified as MB) (8).
It is well known that, on average, the BI will be reduced by a factor of approximately 0.6 to 1 log per year after the start of antileprosy treatment (16,18). Even when the skin smear is still positive after 24 doses of WHO/MDT MB and the patient is released from treatment, this decline will continue (11 and Li, H.-Y. Technical and operational problems in implementing multidrug therapy at different levels. Consultation on technical and operational aspects of MDT in leprosy. Maldives, 1990). Our results are comparable with this. Since the relapse rate after a WHO/MDT MB regimen of 24 doses has proved to be extremely low, the WHO has recommended that MB patients be treated with a fixed regimen of 24 doses instead of until attaining skin-smear negativity (19). Some doubts have recently been cast on this new policy. It was found that patients with a pretreatment BI of 4+ or more had a significantly higher risk of relapse than did patients with an initial lower BI, and it was suggested that the follow up after RFT should be at least 10 years (11,16).
Clinical and histopathological activity. Clinical and histopathological activity/inactivity at RFT and thereafter (Tables 4-6) had no bearing on the final outcome, namely, the relapse rate. The two PB patients who relapsed 5.5 and 7.5 years after RFT had no signs of clinical/pathological activity at the time of RFT and RFS. At RFT more patients showed signs of histopathological activity than clinical activity (Table 5). Most clinically active PB patients became inactive within 2 years after RFT. These data are comparable with those from Ethiopia (3). We observed also a delay of the histopathological cure compared with the clinical one (5). In a study in India small lepromatous granuloma was seen to persist after RFT, and its presence was not found to have any significant association with the duration of the disease or length of treatment (7).
Late reactions. Late reactions were a new phenomenon in the 1980s after the introduction of the WHO short-course regimen. Nowadays guidelines are provided to differentiate between a late reaction and a relapse(2). Even so, this remains very difficult. Late reactions, mild and severe, happen mostly within the first 3-4 years after RET.
Impairments. This study did not aim to look specifically into the appearance of reactions and their treatment or the impairment and the prevention of disabilities (POD). Only since 1987, with the introduction of the POD program, has more attention been paid to early detection and treatment of nerve damage and the teaching of self care to the patients.
CONCLUSION
The fixed-duration WHO/MDT regimens in PB (six doses within 9 months) and in MB patients (24 doses within 36 months) are effective, acceptable and safe, and have an extremely low relapse rate even after many years of follow up. Skin-smear positivity, clinical and histopathologic] activity at RET have no impact on the efficacy of the WHO/MDT regimens and, therefore, there is no need to continue WHO/MDT when one of those signs is still present at RFT. From our study we cannot rebut or conclude that skin-smear-negativc BT patients with multiple lesions have a higher risk of relapse when given WHO/MDT PB. Classification (PB or MB) of these patients should be undertaken only after careful consideration of all clinical features (16). It is not clear yet if the risk of a relapse in patients with a high initial BI will be higher than now anticipated by the WHO. This study, with an average follow up of 8 years among MB patients with a BI of 4+ or more, suggests that this is not the case. In most studies the follow-up period has been too short; follow-up periods of at least 10 years arc advised.
If the WHO PB/MB classification for control programs based on clinical and bacteriological criteria is considered the "gold standard," then a classification system based on clinical criteria only will most likely lead to higher PB relapse rates (due to false-negative MB) and higher costs (due to overclassification; false-positive MB cases) for the program. When and where possible reliable skin-smear services should be maintained. It could be argued that the cost of maintaining skin-smear service should be balanced by the extra costs of overclassification.
The different criteria used to differentiate between a late reaction and a relapse lead lo higher reported relapse rates in some programs. New activity during the first 3-4 years after RFT is mostly due to a late reaction. The longer the period alter RFT the higher the chance that signs of new clinical activity signify a relapse.
Even though the WHO/MDT regimens proved to be very effective in preventing relapses, the outcome of patient management regarding new/additional impairments was less satisfactory. Prevention of disability (POD) activities should be part and parcel of any MDT program from the beginning. When reporting on the outcome of the treatment regimen for leprosy not only the longterm outcome of the chemotherapy but also the long-term outcome of case management, i.e., the proportion of patients with new/additional impairments while on treatment and thereafter, should be taken into account and reported.
Acknowledgment. The Thailand Ministry of Public Health kindly allowed us to publish the outcome of this study. We wish to acknowledge the important contributions made by the staff of the Leprosy Control Centre Region 6, Khon Kaen, and the staff of the Provincial Leprosy Control Offices in Mahasarakham, Roi-et and Kalasin. We want to mention especially Mr. Piya Piyasilpa, previous Director, LCC Region 6, and Ms. Prakong Thiabsri, Leprosy Supervisor, LCC Region 6. To Dr. Marijke Becx, who kindly read an earlier draft of this article and encouraged us to continue with the task, we are grateful. We wish to extend our warm thanks to Dr. Wim H. van Brakel for critically reading this article and for his many valuable comments and suggestions.
REFERENCES
1. BECX-BLEUMINK, M. Allocation of patients to paucibacillary or multibacillary drug regimens for the treatment of leprosy: a comparison of methods based on skin smears as opposed to clinical methods; alternative clinical methods for classification of patients. Int. J. Lepr. 59(1991)292-303.
2. BECX-BLEUMINK, M. Relapses among leprosy patients treated with multidrug therapy: experience in the Leprosy Control Program of the All Africa Leprosy and Rehabilitation Training Center (ALERT) in Ethiopia; practical difficulties with diagnosing relapses; operational procedures and criteria for diagnosing relapses. Int. J. Lepr. 60(1992)421-435.
3. BECX-BLEUMlNK, M. Duration of multidrug therapy in paucibacillary leprosy patients: experiences in the leprosy control program of the All Africa Leprosy and Rehabilitation Training Center (ALERT) in Ethiopia. Int. J. Lepr. 60(1992)436-444.
4. BECX-BLEUMINK, M. and BERHE. D. Occurrence of reactions, their diagnosis and management in leprosy patients treated with multidrug therapy; experience in the leprosy control program of the All Africa Leprosy and Rehabilitation Training Center (ALERT) in Ethiopia, Int. J. Lepr, 60(1992)173-184.
5. BOER RIGTER, C ., PONNIGHAUS, J. M., FINE, P. E. M. and WILSON, R. J. Four-years follow up results of a WHO-recommended multiple-drug regimen in paucibacillary leprosy patients in Malawi. Int. J. Lepr. 59(1991) 255-261.
6. DE RUK, A. J., GABRE, S., HVASS. I', and BERHANU, T. Field evaluation of WHO-MDT of lixed duration, at ALERT, Ethiopia: the AMFES project-I. MDT course completion, case-holding and another score for disability grading. Lepr. Rev. 65(1994)305-309.
7. DESIKAN, P. and DESIKAN, K. V. Persistence of lepromatous granuloma in clinically cured cases of leprosy. Int. J. Lepr. 63(1995)417-421.
8. GROENEN, G., SAHA, N. G., RASHID, M. A., HAMID. M. A. and PATTYN, S. R. Classification of leprosy cases under field conditions in Bangladesh. 1. Usefulness of skin smear examinations. Lepr. Rev. 66(1995)126-133.
9. GROENEN, G., SAHA, N. G., RASHID, M. A., HAMID, M. A. and PATTYN, S. R. Classification of leprosy cases under field conditions in Bangladesh. II. Reliability of clinical criteria. Lepr. Rev. 66(1995)134-143.
10. JAMET, P., JI, B. and THE MARCHOUX CHEMOTHERAPY STUDY GROUP. Relapse after long-term follow up of multibacillary patient reated by WHO multidrug regimen. Int. J. Lepr. 63(1995)195-201.
11. PATTYN, S. R., BOURLAND, J., GRILLEONE, S., GROENEN, G. and GUYS, P. Combined regimen of one year in the treatment of multibacillary leprosy-I. Combined regimens with rifampicin administered during one year. Lepr. Rev. 60(1989)109-117.
12. PIRAYAVARAPORN, C. and PEERAPAKORN, S. The measurement of the epidemiological impact of multidrug therapy. Lepr. Rev. 63 Suppl.(1992) 84s-92s.
13. ROSE, P. and WATERS, M. Reversal reactions in leprosy and their management. Lepr. Rev. 62(1992)113-121.
14. VAN BRAKEL, W. H. DE SOLDENHOFF. R. and MCDOUGALL., A. C. The allocation of leprosy patients into paucibacillary and multibacillary groups for multidrug therapy, taking into account the number of body areas affected by skin, or skin and nerve lesions. Lepr. Rev. 63(1992)231-246.
15. WATERS, M. F. R. Relapse following various types of multidrug therapy for multibacillary leprosy. Lepr. Rev. 66(1995) 1-9.
16. WHO EXPERT COMMITTEE ON LEPROSY. Sixth Report. Geneva: World Health Organization, 1988. Tech. Rep. Ser. 765.
17. WORLD HEALTH ORGANIZATION. A guide to leprosy contro l. 2nd edn. Geneva: World Health Organization, 1988.
18. WORLD HEALTH ORGANIZATION. Chemotherapy of leprosy for control programmes. Geneva: World Health Organization, 1982. Tech. Rep. Ser. 675.
19. WORLD HEALTH ORGANIZATION. Chemotherapy of leprosy. Geneva: World Health Organization, 1994. Tech. Rep. Ser. 847.
20. WORLD HEALTH ORGANIZATION. The Leprosy Unit, Division of Tropical Diseases. Risk of relapse in leprosy. Geneva: World Health Organization, 1994. WHO/CTD/LEP/94.1.
1. M.D., Leprosy Division, Ministry of Public Health, Soi Bamrasnaradoon Hospital, Tiwanond Road, Nonthaburi 11000. Thailand.
2. M.D., M.P.H., Leprosy Division, Ministry of Public Health, Soi Bamrasnaradoon Hospital, Tiwanond Road, Nonthaburi 11000. Thailand.
3. M.D., M.Sc, Leprosy Control Centre Region 6, Khon Kaen, Thailand.
Received for publication on 23 September 1996.
Accepted for publication in revised from on 6 February 1997.