- Volume 65 , Number 1
- Page: 37–44
Daily multidrug therapy for leprosy; results of a fourteen-year experience
ABSTRACT
Between 1980 and 1994, 67 new or relapsing leprosy patients were treated by daily administered multidrug regimens. Tuberculoid patients (23 TT/BT) received either bithcrapy [rifampin+dapsone or clofazimine (RMP+DDS or CLO)]or tritherapy [RMP+DDS and/or CLO and/or ethionamide (ETH)] until clinical cure. Lepromatous patients (44 BB/BL/LL) received tritherapy (RMP+DDS and/or CLO and/or ETH) at least until bacteriological negativity. Of the 23 tuberculoid patients only one patient (5%) was cured at 6 months and about 70% needed between 6 and 24 months of treatment to obtain clinical cure (mean 19.5 months). In the 44 lepromatous patients, the achievement of bacteriological negativity was significantly linked to the initial bacterial index (BI), and it occurred after 2 to 7 years (mean 66.5 months) of multidrug therapy (MDT). The average BI decrease per year was 1.1+ during the first year, 0.9+ the second year, and then < 0.5+ per year. Reactional states significantly (p < 0.01) influenced the BI course: reversal reactions (RR) accelerated while erythema nodosum leprosum (ENL) delayed the BI decrease. Three of the 23 (13%) tuberculoid and 19 of the 44 (43%) lepromatous patients (p < 0.02) exhibited a RR and 18 of 44 (41%) lepromatous patients had ENL during MDT. A late RR (LRR) was observed in 1 (5%) and 6 (17%) of our tuberculoid and lepromatous patients, respectively, and 3 (8%) of our lepromatous patients suffered post-MDT ENL. No confirmed relapse has been observed within a follow-up period of 6 months to 7 years and 3 months [59 person-years at risk (PYR)[ for TT/BT patients and of 4 months to 5 years and 10 months (100 PYR) for BB/BL/LL patients. When compared to the recommended WHO/MDT, it appears that daily MDT docs not increase the clinical or the bacteriological cure rates either at 6 months in paucibacillary tuberculoid patients or at 2 years in multibacillary lepromatous patients. Moreover, as does the WHO/MDT, our regimens show a high frequency of reactional states both during and after treatment. This fact constitutes the main new problem of the actual treatment of leprosy.RÉSUMÉ
Entre 1980 et 1994, 67 malades de la lèpre, nouveaux cas ou rechutes, ont été traités par des régimes de polychimiothérapie administrés quotidiennement. Les patients tuberculoides (23 TT/BT) ont reçu soit une bi-thérapie (rifampicine + dapsone ou clofazimine [RMP+DDS ou CLO]) soit une tri-thérapie (RMP+DDS et/ou ethionamide [ETH]) jusqu'à guérison clinique. Les malades lépromateux (44 BB/BL/LL) ont reçu une tri-thérapie (RMP+DDS et/ou CLO et/ou ETH) au moins jusqu'à négativation bactériologique. Parmi les 23 patients tuberculoides, seul un fut guéri à six mois, et environ 70% ont nécessité entre 6 et 24 mois de traitement pour obtenir la guérison clinique (moyenne 19.5 mois). Parmi les 44 patients léproma teux, la négativation bactériologique était positivement liée à l'index bactérien (IB) initial, et est survenue après 2 à 7 ans (moyenne 66.5 mois) de polychimio-thérapie (PCT). Le diminution annuelle moyenne de l'IB était de 1.1+ au cours de la première année, de 0.9+ au cours de la deuxième année, et ensuite in férieure à 0.5+ par an. Les états réactionnels ont signilicativement influencé (p < 0.01) l'évolution de l'IB: les réactions réverses (RR) ont accéléré, tandis que l'érythème noueux lépreux (ENL) a ralenti la diminution de l'IB. Trois des 23 (13%) tuberculoides et 19 des 44 (43%) de malades lépromateux (p < 0.02) ont montré une RR et 18 des 44 (41%) des malades lépromateux ont eu un ENL durant la PCT. Une réaction réverse tardive a été observée chez, respectivement 1 (5%) el 6 (7%) de nos patients tuberculoides el lépromateux, et 3 (8%) tie nos patients lépromateux ont souffert d'un ENL après la PCT. Aucune recluite confirmée n'a été observée durant une période de suivi allant de 6 mois à 7 ans et 3 mois (59 personnes-années à risque [PAR]) pour les patients TT/BT et de 4 mois à 5 ans et 10 mois (100 PAR) pour les patients BB/BL/LL. Quand on le compare à la PCT recommandée par 1'OMS, il apparaît que la PCT quotidienne n'augmente pas les taux de guérison clinique ou bactériologique à 6 mois chez les patients tuberculoides paucibacillaires et à 2 ans chez les patients lépromateux multibacillaires. De plus, ainsi que la PCT recommandée par l'OMS, nos régimes montrent une grande fréquence d'états réactionnels durant et après la traitement. Ce fait constitue le problème principal du traitement actuel de la lèpre.RESUMEN
Entre 1980 y 1994 se trataron 67 casos nuevos o recurrentes de lepra con poliquimioterapia diaria. Los pacientes con lepra tuberculoide (23 TT/BT) recibieron biterapia (rifampina + dapsona o clofazimina (RMP+DDS o CLO) o triterapia (RMP+DDS y/o etionamida, ETH) hasta su curación clínica. Los pacientes lepromatosos (44 BB/BL/LL) recibieron triterapia (RMP+DDS y/o CLO y/o ETH) cuando menos hasta su negativización bacteriológica. De los 23 pacientes tuberculoides sólo un paciente (5%) fue curado a los 6 meses pero el 70% de los pacientes necesitaron entre 6 y 24 meses de tratamiento para alcanzar la cura clínica (media 19.5 meses). En los 44 pacientes lepromatosos, la negativización bacteriológica estuvo significativamente ligada con el índice bacteriano inicial (IB) y ocurrió después de 2 a 7 años (media 66.5 meses) de poliquimioterapia (PQT). La disminución anual promedio en el IB fue de 1.1+ durante el primer año, 0.9+ en el segundo año, y después, < 0.5+ por año. Los estados reaccionales influyeron significativamente (p < 0.01) en el curso del IB: las reacciones reversas (RR) aceleraron la disminución en el IB, el eritema nodoso leproso la retardó. Durante la PQT, 3 de los 23 (13%) pacientes tuberculoides y 19 de los 44 (437o) pacientes lepromatosos (p < 0.02) exhibieron alguna RR, mientras que 18 de los 44 (41%) pacientes lepromatosos tuvieron ENL. Se observó una reacción RR tardía (RRT) en I (5%) y 6 (17%) de los pacientes tuberculoides y lepromatosos, respectivamente, y 3 (8%) de los pacientes lepromatosos presentaron ENL post-PQT. No se observaron recaídas durante un periodo de seguimiento de 6 meses a 7 años y 3 meses (59 personas-años en riesgo, PAR) entre los pacientes TT/BT y de 4 meses a 5 años y 10 meses (100 PAR) entre los pacientes BB/BL/LL. Cuando se compararon con la PQT recomendada por la OMS, las dosis diarias de PQT usadas en este estudio no parecieron incrementar las tasas de curación clínica o bacteriológica a los 6 meses, ni en los pacientes tuberculoides paucibacilares ni en los pacientes lepromatosos multibacilares. Además, como ocurre con la PQT/OMS, nuestro esquema de tratamiento muestra una alta frecuencia de estados reaccionales tanto durante como después del tratamiento. Este hecho constituye el principal problema del tratamiento actual de la lepra.In 1980, at the Saint-Louis Hospital in Paris, the main leprosy center in France, we started to treat leprosy patients with daily antibacillary multidrug regimens according to their tuberculoid or lepromatous status. Our schedules mainly differ from the multidrug therapy recommended in 1982 by the World Health Organization Expert Committee on Leprosy (WHO/MDT) (24) by the daily administration of rifampin and by our criteria of healing judged as necessary to stop the treatment.
We report here the efficacy and the safety of our daily multidrug regimens after 14 years of experience.
PATIENTS AND METHODS
Patients
Sixty-seven leprosy patients diagnosed and followed up between 1980 and 1994 were included in this study. The patients, 48 men and 19 women aged from 2 to 69 years, came from the West Indies (23 cases), Asia or India (16 cases), Africa (18 cases) or Europe (10 cases).
The pretreatment assessment consisted of clinical examination, histology of a cutaneous lesion, the lepromin skin test and a bacteriological load evaluation.
The patients were then diagnosed according to the Ridley and Jopling classification (18) as polar tuberculoid (TT, 3 cases), borderline tuberculoid (BT, 20 cases), midborderline (BB, 2 cases), borderline lepromatous (BL, 24 cases) and polar lepromatous (LL, 18 cases).
Of the 67 patients, 53 had never been treated, 9 had previously received monotherapy with sulfones, and 5 had had two drugs. Fourteen patients (2 BT, 6 BL and 6 LL) were in relapse. Of the 20 BT patients, 14 had moderate-to-severe involvement of one or more nerves before treatment. Among the lepromatous patients, 3 had primary dapsone (DDS) resistance, 4 secondary DDS resistance and 1 had both DDS and rifampin (RMP) resistance. At the onset of treatment, 6 lepromatous patients suffered from erythema nodosum leprosum (ENL) and 4 patients (2 BL and 2 BT) had a spontaneous reversal reaction (RR).
Methods
The patients were allocated to the following regimens:
TT and BT patients. The TT and BT patients were randomly divided into two groups: Group A ( 13 cases) received daily administration of two drugs-RMP (600 mg) plus DDS (100 mg) or clofazimine (CLO; 100 mg). CLO was chosen in case of symptomatic neurologic involvement. Group B (10 cases) initially received daily administration of three drugs-RMP (600 mg) plus two drugs from among DDS (100 mg), CLO (100 mg) or ethionamide (ETH, 250 mg) and, after a mean of 10 months, daily administration of two drugs (RMP+ DDS or CLO). There were no significant differences between the initial status [number of skin lesions, neurological involvement, bacterial index (BI)] of group A or group B patients. All of the patients were treated until clinical cure.
After clinical cure in the two groups, half of the patients received CLO (100 mg) daily, then every 2 days, then twice a week, then once a week for a mean of 12 months to try to prevent the occurrence of a late reversal reaction (LRR).
BB, BL and LL patients. Lepromatous patients received daily administration of RMP (600 mg) plus two drugs from among DDS (100 mg), CLO (100 mg) or ETH (250 mg). Of the 44 cases, 22 received RMP+DDS+CLO, 14 received RMP+ ETH+CLO, and 8 received RMP+ETH+ DDS. Patients in relapse after sulfone monotherapy received RMP+ETH+CLO as did patients with nerve involvement or ENL. MDT has been given at least until smear negativity and, in some cases, up to 2 years after bacteriological negativity. At the end of MDT, as in tuberculoid patients, about half of the patients received decreasing doses of CLO for a mean of 16 months.
Survey during treatment
During MDT, the patients were examined every 3 months for clinical evaluation and for signs of type 1 (RR) or type 2 (ENL) reactional states. During reactional states MDT was never stopped. Severe RR was treated by prednisone (1 mg/kg/day), mild RR by acetylsalicylic acid (2 to 3 g/day), and pure cutaneous RR by topical steroids. For ENL, patients received either thalidomide (starting at 400 mg/day) or acetylsalicylic acid (2 g/day) or prednisone (0.5 mg/kg/day). Neurolysis was performed in case of failure of medical treatment.
In the absence of reactional states, bacteriological evaluation was done every 6 months in BB, BL and LL patients. A routine biological survey included, at months 1, 2, 3, and 6, white and red blood cells counts, hemoglobin, methemoglobin, bilirubin, alkaline phosphatase, aspartate transaminase, alanine aminotransferase and gamma glutamyl transpeptidase serum levels.
Survey after treatment
After completion of MDT, the patients were followed up every 6 months for, respectively, 5 years for TT and BT patients and 7 to 10 years for BB, BL and LL patients with a bacterial load evaluation once a year.
A relapse was diagnosed as the slow reappearance of new cutaneous lesions. For BB, BL and LL cases, a positive BI at sites previously negative and a still negative lepromin test were also needed for the diagnosis.
Statistical analysis
We used the χ2 test Yates's corrected χ2 test, and the Mann-Whitney U test.
RESULTS
Course during MDT
TT and BT patients (Fig. 1 A). A study of the 23 TT and BT cases showed that only 1 patient was clinically cured at 6 months. This rate increased to 6 patients (26%) after 12 months, 10 (43%) at 18 months, 17 (73%) at 24 months, 20 (86%) at 36 months, and all at 40 months. The mean duration was 19.5 months and 69% of the patients needed 6 to 24 months of treatment. No significant difference was found between Groups A and B at the time of clinical clearing (19 months for Group A versus 21 months for Group B). Moreover, in Group A, no difference in the time for clinical cure was noted between RMP+DDSand RMP+CLO-treated patients (U test; not significant).
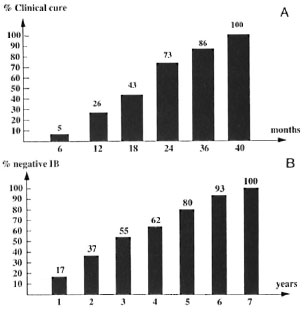
Fig 1. A. Percentage of cinically cured tuberculoid patients by months of daily MDT. B. Percentage of negative lepromatous patients by year of daily MDT.
The two relapsed BT patients were judged cured at 10 and 16 months, respectively. The time for clinical cure for BT patients without neuritis (6 cases) or with neuritis (14 cases) was, respectively, 16.3 and 25.3 months. Of the 12 patients who had nerve palsy before treatment, eight did not improve after treatment. No nerve palsy occurred in patients free of it at the onset of MDT.
BB, BL, LL patients. Of the 44 BB, BL, LL cases, 5 were lost during treatment and 3 are still under MDT. The patient with both RMP- and DDS-resistant bacilli was initially treated in 1982 by RMP+ETH+ CLO. When the results of the mouse foot pad inoculation became known, he received ETH+CLO for 3 months and then was lost to treatment. In our patients the wide polymorphic clinical involvement made difficult the evaluation of the therapeutic efficacy only on clinical improvement. So, as many authors, we chose the bacteriological load decrease to evaluate the therapeutic response.
Morphological index (MI). Before treatment, 78% of the patients had a positive MI (range 2% to 80%, with an average of 39%). No statistical difference existed between the mean MI of the 12 relapsed patients and the never-treated patients. After 12 months, 82 % of the patients had a negative Ml and the still positive patients had a Ml of < 2% . The patients with a still positive MI had the highest MI at the onset of treatment, ranging from 53 % to 80% . After 18 months, all of the patients had a negative MI.
Bacterial index (BI). The mean BI value before treatment was 3.4+ . There was no statistical difference between the mean Bl of the relapsed patients and the nevertreated patients (3.57+ vs 3.36+, p > 0.1). Under MDT it fell to 2.2 + at 12 months, to 1.3 + at 24 months, and then more slowly to 1.1+ at 3 years, 0.6 + at 4 years, 0.5 + at 5 years, 0.3+ at 6 years, and 0.04+ at 7 years.
The mean decrease has been 1.1 + during the first year of treatment, 0.9 + during the second year, and then 0.3 + every year. Of the 29 cases treated by three drugs until reaching a negative BI, five (17% ) patients were negative after I year. This rate increased to 1 1 (37%) at 2 years, 16 (55 % at 3 years, 18 (62%) at 4 years, 23 (80% at 5 years, 27 (93% ) at 6 years and all at 7 years; 16 (55%) needed 2 to 6 years to achieve a negative BI (Fig. 1B). The mean duration of treatment has been 66. 5 months, ranging from 1 to 7 years. The mean time for BI negativity was 80 months for the relapsed patients and 64 months for the never-treated patients (p = 0.052 , not significant).
A statistical correlation exists between the initial BI values and the time needed to obtain bacterial negativity. After 4 years of treatment, 15/2 2 patients who had an initial BI of > 3+ but only 5/1 7 patients who had an initial BI of < 3+ were still positive (p < 0.01).
Reactional states during MDT. Reactional states significantly influenced the course of the BI. The occurrence of RR accelerated BI negativity, e.g., at 3 years of MDT, 13/1 9 (68% ) of BI-negative patients had had RR during MDT versus only 1/22 (5% ) of still Bl-positive patients (p < 0.001) . On the other hand, the occurrence of ENL was associated with a delayed BI negativity, e.g., at 3 years of MDT, 2/11 (18% ) of BI-negative patients versus 16/20 (80% ) of still BI-positive patients had had ENL (p < 0.01) . Figure 2 emphasizes for each patient the relationship between a reactional state and the delay in becoming BI negative.
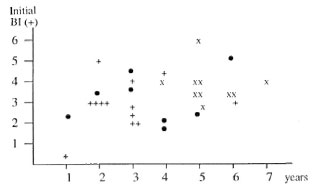
Fig. 2. Time until BI negativity of lepromatous patients with or without reactional states (reversal reaction or ENL). • = Without RR or ENL; + = with RR; x = with ENL.
Reversal reactions. During treatment, RR were observed in 22 of the 67 patients, 3 tuberculoid (13% ) and 19 (43% ) lepromatous patients (p < 0.02) . Patients often had several episodes (2 2 patients had 31 episodes). RR occurred after a mean of 15 months (II to 19 months) in tuberculoid and 19 months (15 days to 7 years) in lepromatous patients, respectively. The medical treatment alone was not sufficient in seven patients who needed neurolysis.
ENL. Eighteen (41% ) lepromatous patients suffered from ENL; 6 (13% ) of whom had had tin ENL reaction before the onset of MDT. Chronic or recurrent EN (duration 6 to 60 months) was observed in 11 patients.
As an average, the first episode of ENL occurred after 15 months of treatment (range 2 months to 4 years). The mean pretreatment BI value was significantly correlated with the risk of ENL. Among 10 patients who had an initial BI of < 3+ only 1 (10%) developed ENL versus 17/30 (56%) patients who had an initial BI of > 3+ (p < 0.05). Medical treatment was sufficient in 15 patients and three cases needed nerve surgery. The evolution has always been favorable without neurologi sequelae.
Drug toxicity. CLO-induced skin pigmentation and dryness occurred in 80% of the patients. Except for one case, this side effect did not necessitate CLO withdrawal.
Twelve episodes of hepatitis o ccurred in 9 patients [3 (13%) tuberculoid and 6(13%) lepromatous]. All cases were asymptomatic and were diagnosed on increased serum levels of hepatic enzymes more than twice the upper limit of normal. Nine episodes were due to ETH, corresponding to 28% of the 32 patients who received this drug. The mean duration between the onset of MDT and ETH-induced hepatitis was 5 months. Withdrawal of ETH rapidly resulted in biological recovery in 8 of 9 patients. In the last case, biological abnormalities persisted despite cessation of ETH and a return to normality required the cessation of both ETH and RMP. In two patients, viral hepatitis B infection as the etiologic or coprecipitating factor could not be excluded. One patient developed an autoimmune hepatitis induced by RMP 40 months after the onset of MDT and then, 37 months later, an ETH-induced hepatitis. One patient has had hepatitis due to DDS.
DDS-induced hemolytic anemia occurred between 8 days and 12 months after the onset of MDT (mean 4.8 months) in six lepromatous patients (17% of cases receiving DDS) but in none of the TT or BT patients. The hemoglobin level never decreased under 8 g/100 ml. No patient had G-6-P-D (gIucose-6-phosphodehydrogenase) deficiency. The anemia has been well-tolerated, and the hemoglobin levels returned to normal after cessation of MDT in all except three patients for whom DDS had to be immediately stopped. Of these 3 patients, 1 also had an eosinophilia and an increased gamma glutamyl transpeptidase serum level. 1 a leucopenia, and 1, severe methemoglobinemia. All of these disturbances disappeared after DDS withdrawal.
An RMP-induced flu syndrome occurred in one case without detectable circulating anti-RMP antibodies.
Course after MDT
BT and TT patients. Twenty-one of 23 patients have been followed up for a mean of 34 months (range 6 months to 7 years and 3 months). No confirmed relapse has been observed. In one case, we could not distinguish between a persistent cutaneous lesion or relapse: this patient received 13 months of MDT and treatment was stopped in spite of a still slightly visible cutaneous lesion which was unchanged 12 months later. Histology was still that of BT leprosy. We decided to treat again and an additional 6 months of MDT were necessary to cure the lesion. After 3 years of follow up, the patient remains clinically cured.
BB, BL and LL patients. Among the 44 patients, 6 have been lost to control during or at the end of treatment, 3 are still being treated, and 35 patients have been followed up for a mean of 34 months (range 4 months to 5 years and 10 months). Of these, 29 have been followed for > 1 year (mean 40 months), 17 for > 3 years (mean 52.5 months), 1 1 for > 4 years (mean 57 months) and 5 for > 5 years (mean 65 months). No relapse has been observed.
Reactional states. Late reversal reactions (LRR). Seven patients had LRR [ 1 BT (5%) and 6 BL (17%)]. The BT patient had LRR 10 days after cessation of treatment; the lepromatous patients' LRR occurred at a mean of 15 months after treatment (range 3 to 43 months). Systemic steroids were sufficient and there were no nerve sequelae.
The differential diagnosis between LRR and relapse has been difficult, especially in the first cases of LRR, and comparisons of clinical photographs, histology, bacteriology and the lepromin skin test before MDT and at the time of LRR were useful. It appears that our criteria for stopping the treatment, clinical cure in tuberculoid patients, and bacteriological negativity in lepromatous patients, facilitated the discussion.
In order to prevent LRR, 10 tuberculoid and 15 lepromatous patients after MDT received decreasing doses of CLO for a mean of 12 and 16 months, respectively. Among these patients, 3 LRR (3 BL) have been observed versus 4 (1 BT and 3 BL) among the 34 patients without CLO. There is no significant difference, but this sample is small.
Post-MDT ENL. Clinically subacute ENL continued to occur in three (8%) lepromatous patients during the followup period and persisted for 1, 10 and 13 months, respectively. These patients had had chronic ENL during MDT. The Bl was always negative. Medical treatment alone was sufficient.
DISCUSSION
We started this study in 1980, 2 years before the first WHO recommendation for leprosy treatment with WHO/MDT. Our schedules mainly differ from that of the WHO by the daily administration of rifampin (RMP) and by the duration of treatment. At the time of sulfone monotherapy, in BI-negative or low-positive tuberculoid patients, while the bacterial load decrease could not be used most leprologists took as cure the criteria of the disappearance of the cutaneous lesions. In multibacillary (MB) lepromatous patients, bacteriological criteria (MI and BI negativity) have always been used to evaluate the efficacy of therapy (23). For the above reasons, we decided to treat tuberculoid patients until clinical clearing and lepromatous patients until BI negativity.
Between 1980 and 1994 we treated 67 new or relapsing leprosy patients with a daily MDT of either RMP+DDS or RMP+ CLO or RMP+DDS+CLO in tuberculoid cases and RMP+DDS and/or CLO and/or ETH in lepromatous patients.
In tuberculoid patients, our results show that an average duration of 19.5 months of treatment is necessary to achieve clinical cure. Thus, if most patients (70%) are cured after 6 to 24 months of treatment, a few others (10%) needed up to 40 months for clearing. Only one patient (5%) was cleared at 6 months. In PB patients treated with WHO/MDT for 6 months as recommended, widely varying percentages of clinical cure have been reported, mainly < 50% up to 90%-95% in two studies (5-l7). Our study shows that daily RMP either in two or three drug combinations does not increase the cure rate at 6 months and that RMP+CLO or RMP+DDS are quite as potent.
Yet, if currently almost all leprologists agree that in PB tuberculoid patients the complete clearing of skin is not necessary to stop treatment with MDT (25), in our experience it appears that it may be very difficult to differentiate among a persistent lesion, a relapse and a reversal reaction (7, 13, 14, 16, 25) if treatment is stopped before the clearance of the lesions.
In our lepromatous patients, the MI rapidly decreased to < 5% within the first 6 months. MI negativity occurred within the first 12 months in > 80% of the cases and the still MI-positive cases exhibit a low (l%-2%) MI value.
BI negativity after 2 years of MDT has been obtained in only 37% of the cases, and 55% of the cases needed from 2 to 6 years of treatment to become negative. This wide range reflects the statistical correlation between the initial BI level and the time for bacterial clearing. The average decrease per year is about 1+ during the first 2 years and then is < 0.5+ per year. With WHO/MDT, at the end of 2 years of treatment variable percentages of BI-negative patients have been reported, from 0 up to 64% (4,10), and the mean BI decrease is about 0.6+ per year for the first 2 years (9). Our results show that daily MDT does not increase the bacteriological cure at 2 years. It could induce a more rapid BI decrease during the first 2 years, but this effect would have needed a control group on monthly RMP in our study to be confirmed. It is well known that many MB patients, especially those with an initial BI of > 4+, are still smear positive at 24 months of WHO/MDT may later achieve negativity without further chemotherapy. It is highly probable that the same feature would occur with our MDT and, perhaps, because of this initially faster BI decrease, in a greater number of cases.
The reactional states appeared to have a statistical influence on the bacterial load decrease: in BL and LL patients, RR accelerates while ENL slows down the BI decrease. This finding, rarely noted in the published data on WHO/MDT, strengthens the usefulness of a bacteriological survey during the months following these episodes.
In addition to their effects on the bacteriological clearing, the reactional states worsen the prognosis because of the severe damage they may induce (1, 6, 11, 20). In our study, RR during therapy occurred in 13% of the tuberculoid and 43% of the lepromatous patients, most of them appeared during the first and second year of treatment, respectively. Neurological sequelae despite medical treatment and surgery have been noted in 27% of cases. These results are not different from those reported with WHO/ MDT: 0 to 19% of RR in paucibacillary (PB) (12, 15) and 16% to 45% in multibacillary (MB) patients (3, 19) with a 38% incidence of sequelae (11).
Forty-one percent of our lepromatous patients exhibited ENL. No nerve damage has been noted. Our frequency of ENL seems greater than that reported (30%) with the WHO/MDT (3, 19, 21). This may result from a greater bactericidal effect of RMP given daily (8). Since ENL is assumed to be an immune complex syndrome, it is possible that daily RMP induced greater and more abrupt releases of Mycobacterium leprae antigens than monthly RMP. It may also be related to the fact that about 20% of our patients do not receive CLO in their regimens.
With the introduction of MDT prescribed for a limited period, a new problem has been raised for the leprologists: the occurrence of RR after cessation of treatment. In our study, LRR were observed in 5% and 17% of tuberculoid and lepromatous patients, respectively. After WHO/MDT, LRR occurred in 1% to 16% (2, 5) of PB and in 1% to 37% (2, 22) of MB patients. As in our study, they occurred in the first 6 months after completion of MDT in PB tuberculoid patients and later, between 15 and 18 months, in MB lepromatous patients.
To try to prevent LRR, 10 tuberculoid and 15 lepromatous patients received CLO with decreasing doses for a mean of 13 and 16 months, respectively. Among these 25 patients, 3 LRR (in 3 BL cases) have been observed versus 4 (1 BT and 3 BL) among 34 patients without CLO. There is no significant difference, but the number of patients is small. Perhaps with a larger trial group a significant difference would have been found.
The persistence of ENL after MDT has never been reported; however it has been observed in 8% of our lepromatous patients. All had had ENL during MDT, all were BI negative, and all responded well to anti-ENL medical treatment alone.
Except for the unacceptable high incidence of hepatitis in patients taking ETH (28% of ETH-treated patients), our MDT has been well tolerated. Because of this liver toxicity, 6 years ago we stopped the use of ETH which, moreover, is no longer available in France. Mild side effects, mainly DDS-induced, well-tolerated anemia, were noted in 17% of DDS-treated patients. Severe side effects (allergy to RMP and serious hematologic or hepatic disturbances due to DDS) necessitating cessation of the drug occurred in 10% of our lepromatous patients.
Despite our prolonged duration of treatment, the 100% compliance by tuberculoid patients and 89% by lepromatous patients was satisfying.
No confirmed relapse has been observed during follow ups of 6 months lo 7 years and 3 months [59 person-years at risk (PYR)] in tuberculoid patients and of 4 months to 5 years and 10 months (100 PYR) in lepromatous cases.
CONCLUSION
Multidrug therapy constitutes real progress in the treatment of leprosy. Our study shows that, in comparison with WHO/MDT and an equal duration of treatment, the daily administration of RMP does not strikingly accelerate either the clinical or the bacteriological cure of tuberculoid and lepromatous patients. It confirms the relatively high incidence of reactional states (RR or ENL) that certainly constitutes the "negative" aspect of MDT. Moreover, it shows that despite our strict criteria the diagnosis of LRR is a real difficulty and constitutes the "new" problem arising with the MDT.
REFERENCES
1. BECX-BLEUMINK, M. and BERHE, D. Occurrence of reactions, their diagnosis and management in leprosy patients created with MDT; experience in the leprosy control program of the All Africa Leprosy and Rehabilitation Training Center (ALERT) in Ethiopia. Int. J. Lepr. 60(1992)173-184.
2. BLOK, L. M., BLOOS, L. J. and VAN DEN BERG, G. A retrospective study of seven years of multiple drug treatment for paucibacillary and multibacillary leprosy in Bayara General Hospital, Nigeria. Lepr. Rev. 62(1991)193-200.
3. CHATTOPADHYAY, S. P., GUPTA, C. M., BHATE, R. D., BHATE, R. P. and SREEVASTA. Evaluation of two multidrug regimens in hospitalised multibacillary cases. Indian J. Lepr. 61(1989)196-205.
4. CHENG, Z.-Q., YANG, L.-H., FAN, D.-H. and HUANG, C.-Y. Results of skin smears from eightyfour MB patients on MDT MB regimen recommended by WHO. (Abstract) Int. J. Lepr. 61(1993)10A(CH 40).
5. DAY, R. S. and TETERISSA, M. R. Field trial of short course combined chemotherapy in paucibacillary leprosy. (Abstract) Int. J. Lepr. 57 Suppl.(1989)340.
6. DE RIJK. A. J., GABRE, S., BYASS, P. and BERHANU, T. Field evaluation of WHO-MDT of fixed duration, at ALERT, Ethiopia: the AMFES project-II. Reaction and neuritis during and after MDT in PB and MB leprosy patients. Lepr. Rev. 65(1994)320-132.
7. FLAGEUL, B., VIGNON-PENNAMEN, M. D., WALLACH, D., PENNEC, J. and COTTENOT, F. Les reactions de reversion tardive au cours de la lèpre. Acta Leprol. 7(1990)109-117.
8. GROENEN, G., JANSSENS, L., KAYEMBE, T., NOLLET, E., COUSSENS, L. and PATTYN, S. R. Prospective study on the relationship between intensive bactericidal therapy and leprosy reactions. Int. J. Lepr. 56(1986)236-244.
9. GROSSET, J. H. Progress in the chemotherapy of leprosy. Int. J. Lepr. 62(1994)268-277.
10. KATOCH, K.. RAMU, G., SENGUPTA, U., SREEVATSA, SHARMA, V. D., SHIVANNAVAR, C. T. and KATOCH, V. M. Results of a modified WHO regimen in highly bacilliferous BL/LL patients. Int. J. Lepr. 57(1989)451-457.
11. LIENHARDT, C. and FINE, P. E. M. Type 1 reaction neuritis and disability in leprosy. What is the current epidemiological situation? Lepr. Rev. 65(1994)9-33.
12. OREGE P. A., OBURA, M., OKELO, C, OKUKU, P., NYAWLO, J. and MOKOKHA, S. Multidrug therapy for treatment of paucibacillary leprosy: the western Kenya experience. (Abstract) Int. J. Lepr. 57 Suppl.(1989)341.
13. PANNIKAR, V., JESUDASAN, K., VIJAYAKUMARAN, P. and CHRISTIAN, M. Relapse or late reversal reaction. Int. J. Lepr. 57(1989)526-528.
14. RAMACHANDRAN, A. and SESHADRI, P. S. Relapse or reversal reaction: the case for a therapeutic trial of steroids. Lepr. Rev. 59(1988)271-272.
15. RAMANAN, R., MANGLANI, P. R., GHORPADE, A. and BHAGOLIWAL, S. K. Follow-up study of paucibacillary leprosy on multidrug regimen. Indian J. Lepr. 59(1987)50-53.
16. RAMU, G. Clinical features and diagnosis of relapses in leprosy. Indian J. Lepr. 67(1995)45-59.
17. RANGARAJ, M. and RANGARAJ, J. Experience with multidrug therapy in Sierra Leone: clinical, operational and managerial analysis. Lepr. Rev. 57 Suppl.(1985)77-91.
18. RlDLEY, D. S. and JOPLING, W. J. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966)255-273.
19. TIWARI, V. D., TUTAKNE, M. A., SINGH, G. and DUTTA, R. K. Multidrug therapy in hospitalised leprosy cases. Indian J. Lepr. 60(1988)71-77.
20. VAN BRAKEL, W. H. and KHAWAS, I. B. Nerve damage in leprosy: an epidemiological and clinical study of 396 patients in west Nepal-part I. Definitions, methods and frequencies. Lepr. Rev. 65(1994)204-221.
21. VAN BRAKEL, W. H., KHAWAS, I. B. and LUCAS, S. B. Reactions in leprosy: an epidemiological study of 386 patients in west Nepal. Lepr. Rev. 65(1994)190-203.
22. VIJAYAKUMARAN, P., MANIMOZHI, N. and JESUDASAN, K. Incidence of late lepra reaction among multibacillary patients after MDT. Int. J. Lepr. 63(1995)18-22.
23. WHO EXPERT COMMITTEE ON LEPROSY. Fourth report. Geneva: World Health Organization, 1970. Tech. Rep. Ser. 495.
24. WHO STUDY GROUP. Chemotherapy of leprosy for control programmes. Geneva: World Health Organization. 1982. Tech. Rep. Ser. 675.
25. WHO STUDY GROUP. Chemotherapy of leprosy. Geneva: World Health Organization, 1994. Tech. Rep. Ser. 847.
1. M.D. Clinique des Maladies Cutanees du Pr. L. Dubertret, Hopital Saint-Louis, Avenue Claude Vellefaux, 75475 Paris 10, France.
2. M.D. Clinique des Maladies Cutanees du Pr. L. Dubertret, Hopital Saint-Louis, Avenue Claude Vellefaux, 75475 Paris 10, France.
3. M.D. Clinique des Maladies Cutanees du Pr. L. Dubertret, Hopital Saint-Louis, Avenue Claude Vellefaux, 75475 Paris 10, France.
4. M.D. Clinique des Maladies Cutanees du Pr. L. Dubertret, Hopital Saint-Louis, Avenue Claude Vellefaux, 75475 Paris 10, France.
5. M.D. Clinique des Maladies Cutanees du Pr. L. Dubertret, Hopital Saint-Louis, Avenue Claude Vellefaux, 75475 Paris 10, France.
Reprint requests to Dr. Flageul.
Received for publication on 29 April 1996;.
Accepted for publication in revised form on 4 November 1996.