- Volume 65 , Number 1
- Page: 45–55
Differential development of CD4 and CD8 cytotoxic T cells (CTL) in PBMC across the leprosy spectrum; IL-6 with IFN-γ or IL-2 generate CTL in multibacillary patients
ABSTRACT
In the present study we evaluated the contribution of CD4 and CD8 T cells to the antigen-specific cytotoxic activity induced by whole Mycobacterium leprae in leprosy patients and normal controls (N) as well as the modulation of this activity by some cytokines. Peripheral blood mononuclear cells (PBMC) f rom N or f rom leprosy patients were stimulated with antigen in the presence or absence of cytokines for 7 days. M. leprae- stimulated PBMC were depleted of CD4 or CD8 antigen-bearing cells and employed as effector cells in a 4-hr [31 Cr ] release assay against autologous M. leprae -pulsed macrophages. Our results demonstrate that both CD4 and CD8 T cells contribute to M. leprae -induced cytotoxic activity, with differences observed inpaucibacillary (PB) and multibacillary(MB) patients. CD8-mediated cytotoxic activity is higher than that of CD4 cells in PB patients, while in MB patients CD4 cytotoxicity is predominant.Our data also demonstrate that the generation of CD4 and CD8 cytotoxic T lymphocytes (CTL) can be modulated differentially by interleukin-4 (IL-4), IL-6, gamma interferon (IFN-γ), or IL-2. Although MB patients developed the lowest CTL response, cytokines such as IL-6 plus IL-2 or 1FN-γ were able to generate both CD4 and CD8 cytotoxic T cells f rom MB patients. In PB patients, IL-6 plus IFN-γ displayed the highest stimulation on CD8 effector cells. Thus, an important role may be assigned to IL-6, together with IL-2 or IFN-γ, in the differentiation of M. leprae -specific CTL effector cells.
RÉSUMÉ
Nous avons évalué dans l'étude présente la contribution des cellules T CD4 et CD8 dans l'activité cytotoxique antigéniquement spécifique induite per le Mycobacterium leprae entier chez des malades de la lèpre et des témoins normaux ainsi que la modulation de cette activité par certaines cytokines. Des cellules mononucléaires du sang périphérique (CMSP) de témoins et de malades de la lèpre ont été stimulées avec l'antigène en présence ou absence des cytokines pendant 7 jours. Les CMSP stimulées par M. leprae ont été débarassées de leurs cellules CD4 oti CD8 porteuses d'antigènes et employées comme cellules effectrices dans un test de libération 4-hr [31Cr] vis-à-vis des macrophages autologues stimulés par M. leprae. Nos résultats démontrent que les cellules T CD4 et CDS contribuent toutes deux à l'activité cytotoxique induite par, M. leprae, avec des différences observées chez les patients paucibacilllaires (PB) et multibacillaires (MB). L'activité cytotoxique des CD8 est plus élevée que celle des cellules CD4 chez les patients PB, alors que chez les patients MB, la cytotoxicité CD4 est prédominante. Nos données démontrent également que la production de lymphocytes T cytotoxiques (LTC) CD4 et CD8 peut être modulée de manière différenciée per l'interleukine-4 (IL-4), l'interleukine-6, ['interferon gamma (IFN-γ), ou I'IL-2. Bien que les patients MB aient montré la plus faible réponse LTC, des cytokines telles que IL-6 plus IL-2 ou l'interféron-yétaient capables d'induire la production de cellules T cytotoxiques CD4 et CD8 chez les patients MB. Chez les patients PB, l'IL-6 plus IFN-γ amontré la stimulation la plus élevée sur les cellules effectrices CD8. En conséquence, un rôle impoltant peut être attrribué à l'IL-6, ainsi qu'à l'IL-2 et à l'IFN-γ dans la différenciation des cellules effectrices LTC spécifiques vis-à-vis de M. leprae.RESUMEN
En el presente estudio evaluamos la contribución de las células T CD4 y CD8 en la actividad citotóxica antígeno-específica inducida por Mycobacterium leprae integral, en pacientes con lepra y en controles sanos (N), así como la modulación de esta actividad por algunas citocinas. Para esto, las células mononucleares de sangre periférica (MN) de personas N o de pacientes con lepra, se incubaron durante 7 días con el antígeno, en presencia o en ausencia de citocinas. Las células MN estimuladas con M. leprae se depletaron de las células CD4 o CD8 y se emplearon como células electoras en ensayos de liberación de "Cr de 4 h, contra macrófagOS parasitados con M. leprae. Nuestros resultados demostraron que tanto las células CD4 como las CD8 contribuyeron a la actividad citotóxica inducida por M. leprae, aunque hubieron diferencias entre los pacientes paucibacilares (PB) y los multibacilares (MB). La actividad citotóxica mediada por CD8 es mayor que la mediada por las células CD4 en los pacientes PB. mientras que en los pacientes MB la actividad citotóxica de las células CD4 es la predominante.Nuestros resultados también demuestran que la generación de linfocitos T citotóxicos CD4 y CD8 (CTL) puede ser modulada diferencialmente por las interleucinas IL-4, IL-6, interferon gamma (IFNγ), o IL-2. Aunque los pacientes MB desarrollaron las respuestas citotóxicas más bajas, la IL-6 más la IL-2 o el IFNγ, fueron capaces de generar células T citotóxicas tanto CD4 como CD8 en los pacientes MB. En los pacientes PB. la IL-6 más el IFNyexhibieron la máxima capacidad estimulante sobre las eélulas efectoras CDS. Asi\ la IL-6, junto eon la IL-2 o el IFNγ, parecen jugar un papel importante en la dilerenciaciôn de las eélulas T citotôxicas especi'ficas para M. leprae.
Cytotoxic T cells (CTL) that lyse macrophages presenting mycobacterial antigens have been identified in both murine and human systems (4, l3, 14, 22, 24). This antigen-specific destruction of target cells may be relevant not only for the eradication of the Mycobacterium leprae reservoir but also, from an immunopathological point of view, for the induction of skin and nerve damage such as those present in leprosy patients.
The relative contribution of T-cell subsets to acquired resistance against intracellular bacteria has been analyzed in different experimental models. In murine tuberculosis a dominant role has been associated with CD4 T cells, with some contribution of CD8 T cells (10, l5). Antigen-specific CD4 cells that secrete a Th1 cytokine pattern have been isolated from patients with leprosy or tuberculosis ( 4, 1, 11). These cells express cytolytic activities directed to antigen-presenting macrophages (4, 21, 32). Human class Irestricted CD8 T cells with reactivity for intracellular bacteria have been isolated less frequently (7), although they have been described in tuberculosis and tuberculoid leprosy lesions (21, 31, 38).
The development of CTL in vitro requires antigen as well as nonspecific factors (26). In previous reports we (34), as well as others (13), have shown that multibacillary (MB) patients developed lower cytotoxicity against autologous macrophages presenting M. leprae or related mycobacterial antigens than did paucibacillary (PB) patients and normal controls (N). The cytokine milieu is thought to influence the immune system toward production of a particular T-cell cytokine pattern, resulting in different effector functions. In intracellular infections, cells expressing the type 1 profile (Th1) activate mononuclear phagocytes converting them into potent effector cells, while type 2 (Th2)dependent activities seem to be of minor importance in the activation of phagocytes. Studies of cytokine expression in leprosy lesions have demonstrated the prevalence of Th2 cytokines in lepromatous leprosy patients and of Th1 cytokines in tuberculoid leprosy (41, 42). Antigen-induced cytotoxic activity can be regulated by some of these soluble factors. We have previously demonstrated that M. leprae- induced cytotoxicity of peripheral blood mononuclear cells (PMBC) could be enhanced in vitro by the addition of cytokines such as interferon gamma (IFN-γ), interleukin-6 (IL-6) or the combination of IL-6 plus IL-2, while the addition of IL-4 downregulated this activity (9).
In the present study we have demonstrated that both CD4 and CD8 cytotoxic T cells contributed to the cytolysis of M. leprae -pulsed autologous macrophages, as was suggested in a previous paper by blocking experiments (34), but differences were observed in PB and MB leprosy patients. We also have analyzed the effect of 1L-4, IL-2, IFN-γ and IL-6 on the cytotoxic function of these lymphocyte subpopulations.
MATERIALS AND METHODS
Patients. Fourteen lepromatous (LL), 1 borderline lepromatous (BL), 2 tuberculoid (TT) and 5 borderline tuberculoid (BT) patients [classified according to Ridley and Jopling (32)] were studied. They were divided into two groups: paucibacillary (TT and BT: 4 women, 3 men; 18-70 years of age) and multibacillary (LL and BL: 8 women, 7 men; 20-68 years of age) patients. All of the patients included in this study were free of other infectious diseases and received multidrug therapy according to the recommendations of the World Health Organization (WHO/MDT). Lepromatous patients undergoing erythema nodosum leprosum (ENL) (LL-ENL) were receiving thalidomide. The PB patient with a reversal reaction (BT-RR) was receiving corticosteroid treatment. Twenty-one BCG vaccinated normal controls (N) (11 women, 10 men; 30-55 years of age) were studied simultaneously.
Mononuclear cells. PBMC were isolated by centrifugation of heparinized blood on a Ficoll-Hypaque density gradient (5). Cells were collected from the interphase and resuspended in RPM1 1640 tissue culture medium (GIBCO Laboratories, Grand Island, New York, U.S.A.) containing gentamycin (85 µ g/ml)and 15% heat-inactivated fetal calf serum (FCS) (GIBCO) (complete medium).
Effector cells for cytotoxicity assays. PBMC (2 x 106 cells/ml) were cultured in Falcon 2063 tubes (Falcon Plastics, Oxnard, California, U.S.A.) at 37ºC in humidified 5% CO2 atmosphere in complete medium with or without 1.8 x 107 M. leprae/ ml (gamma-irradiated M. leprae, kindly provided by Dr. R. J. W. Rees of the National Institute for Medical Research, Mill Hill, England, through the IMMYC bank) in the presence or absence of a) IL-6 (20 U/ml), b) IFN-γ(100 U/ml), c) IL-2 (50 U/ml), d) IL-4 (10 U/ml), e) combinations of IL-6 + IL-2 or IL-6 + IFN -y . All cytokines employed in this work were purchased from Genzyme, Boston, Massachusetts, U.S.A. and were recombinant proteins. On day 7, treated and control cells were washed three times with RPMI 1640, resuspended in complete medium (2 x 106 cells/ml), and tested for cytotoxic activity.
Isolation of CD4- and CD8-depleted effector cells. Cultured mononuclear cells (either M. leprae- induced, cytokine-treated or control cells) were depleted of lymphocytes bearing the CD4 or CD8 antigen by treatment with anti-CD4 or anti-CD8 monoclonal antibody conjugated magnetic beads (Dynal, Inc., Great Neck, New York, U.S.A.). Briefly, 2-4 x 106 mononuclear cells, resuspended in 100 µ l of PBS containing 2% FCS, were mixed with 75 µ l of anti-CD4 conjugated or 40 p\ of anti-CD8 conjugated beads (anti-CD4 and anti-CD8 conjugated beads were supplied as a suspension of 1.4 x 10s beads/ml), and incubated for 45 rain at 2-4ºC. Then, PBS-FCS was added to a final volume of 5 ml and the cell suspensions were placed in a magnetic particle concentrator for 3 min to collect the rosetted cells and the unbound beads. The CD4- and CD8-depleted cells were collected, washed three times, then resuspended with complete medium and tested for their cytotoxic activity. Purity of the CD4 and CD8 populations was verified by flow cytometry analysis.
Target cells. PBMC (5 x 106ml) in complete medium were plated in 24-well Falcon plates and incubated for 2 hr at 37ºC in order to obtain the adherent population. After removing nonadherent cells, cells remaining in the plates (10% of the original cell suspension) were incubated at 37ºC in a humidified 5% CO2 atmosphere for 7 days. For the cytotoxic assays, on day 6 of incubation the cells were pulsed with 1.8 x 107 M. leprae /ml . Macrophages kept under the same conditions but without the addition of antigen were used as controls. Plates were cooled for 2 hr at 4ºC to facilitate the detachment of adherent cells by vigorous pipetting using ice-cold medium. These cells were washed and pellets of 5 to 7x 105 cells were labeled with 100 µ Ci of Na2,51Cr04 (New England Nuclear, Boston, Massachusetts, U.S.A.) by incubation for 1 hr at 37ºC. The cells were washed three times and resuspended in complete medium at 1 x 105 cells/ml.
Cytotoxic assay. Target cells (1 x 104) were seeded into each well of 96-well microtitre plates (Falcon). Effector cells were added in triplicate at different effector-totarget cell ratios in a final volume of 0.2 ml. The plates were centrifuged at 50 x g x 5 min and incubated at 37ºC in 5% CO2 for 4 nr. After centrifugation at 500 x g x 5 min, 100 µ l of supernatants were removed from each well. The radioactivity of the supernatants and pellets was measured in a gamma counter. Results were expressed as percentage of cytotoxicity:

Spontaneous release is the radioactivity released from target cells incubated with complete medium alone. It ranged from 15% to 25%.
Flow cytometry analysis. Fresh mononuclear cells as well as cultured effector cells were labeled with FITC or PE-conjugated monoclonal antibodies, specific for the human leukocyte antigen CD45, CD4 (Leu 3a) or CD8 (Leu 2a) lymphocyte antigens and the macrophage-monocyte antigen CD14 (Leu M3) (Becton, Dickinson & Co., San Jose, California, U.S.A.). Cell-surface phenotype was then determined by flow cytometry using a dual color analysis (Cellquest software) on a FACScan flow cytometer (Becton, Dickinson); 15,000-20,000 events were acquired for each sample; gates were set with respect to the forward- and side-scatter to exclude cell debris. Mouse IgG1/IgG2a FITC/PE -conjugated monoclonal antibodies (γ1/γ2) were included as isotype controls.
Statistics. Comparisons of MB and PB patients and normal controls were performed using the Student's t test. Cytotoxicity values obtained form the different subsets of effector cells of each individual were compared using the Wilcoxon matchedpairs signed rank test.
RESULTS
M. leprae- induced cytotoxic assay from CD4 and CDS T-cell enriched populations.
CD4 and CDS antigen expression on M. lep rae-stimulated and control mononuclear cells was determined in order to assess the nature of the effector cells present in the lymphocyte suspensions after 7 days of culture (Table 1). No differences were observed when M. leprae -stimulated CD4 T cells were compared in MB or PB patients and normal controls (N), while in the case of CD8 T cells the percentage was lower in MB than in PB patients and N, and was not increased by stimulation with M. leprae. Confirming our previous work (V1), MB patients developed the lowest cytotoxic activity when M. leprae -induced mononuclear cells were employed in the cytotoxic assay (% cytotoxicity, x ± S.E.M.: MB = 10 ± 1, MB-ENL = 20±6, PB = 33 ± 3, N = 21 ± 1). In the present report, we analyzed the cytotoxic activity of M. leprae - induced CD8- and CD4-depleted effector cells (CD4 and CD8 cells, respectively). First, the purity of the CD4 and CD8 suspensions was determined: CD8 suspensions contained 67%-80% of CD8+ cells and 5%-10% of CD4+ cells while CD4 contained 77%-94% of CD4+ cells and 2%-6% of CD8+ cells. Similar values were obtained for effector cells purified from MB, PB and N.

Then, we determined the cytotoxic activity of M. leprae -induced CD4 and CD8 effector cells. One representative case of each polar form and one normal control are shown in The Figure. For technical reasons, effector cells were seeded at the E/T ratios of 20:1, 40:1 and 80:1 according to the global cell counts and were not adjusted for individual variations of CD4 or CD8 percentages. Afterward, these E/T ratios were corrected according to the percentage of CD4 and CD8 T cells present in the effector populations obtained from the flow cytometry data. CD4 or CD8 effector cells from MB, PB or N presented different patterns of cytotoxicity despite the diverse E/T ratios employed. In further studies, we only employed the E/T ratio 40:1, and the results are expressed as percentage of cytotoxicity after subtracting the lytic activity of primed effector cells against nonpulsed macrophages.
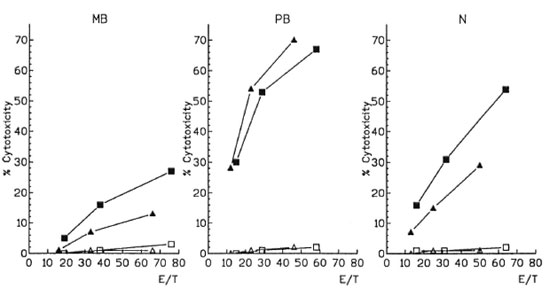
The figure. M. leprae -induced cytotoxicity from CD4 and CD8 effector cells. CD4 (■) and CD8 (▲) effector cells were tested for their cytotoxic activity against autologous antigen-pulsed (■ ▲) and nonpulsed (l  )macrophages at different effector-to-target (E/T) cell ratios. MB = Multibacillary patients; PB = paucibacillary patients; N = controls.
)macrophages at different effector-to-target (E/T) cell ratios. MB = Multibacillary patients; PB = paucibacillary patients; N = controls.
As shown in Table 2, CD4 cytotoxicity was higher than that of CDS in 19 out of 21 controls. A similar pattern was observed in 10/12 MB patients. This difference was most striking in one of the three LL-ENL (% CD4 cytotoxicity = 38%, CD8 cytotoxicity = 8). The cytotoxicity values of both lymphocyte populations of MB were significantly lower than those observed in N (CD4 p < 0.0001, CD8 p < 0.001) and PB patients (CD4 p < 0.01, CD8 p < 0.0001). On the contrary, in 5/7 PB patients CD8 cytotoxicity predominated. Moreover the lytic activity of CD4 was similar to that of N and CD8-mediated cytotoxicity was higher than N in PB (p< 0.001).
In all cases, when macrophage targets were not pulsed with M. leprae (The Figure) or mononuclear cells had not been cultured with M. leprae (data not shown), negligible cytotoxicity was observed (0%-4%), even if cytokines were included in the cultures (l%-6%).
Effect of IL-4, IL-2, IFN-γ and IL-6 on the cytotoxic activity of M. leprae- induced CD4 and CD8 effector cells. IL-4 completely inhibited the generation of CD4 effector cells in MB patients and produced a diminution of CD4 cytotoxicity in PB patients and N (Table 3). CD8 cytotoxicity was abrogated in both MB patients and N, while the inhibitory effect was less evident on CD8 cytotoxic activity from PB patients.
When we analyzed the cytotoxic activity of CD4 and CDS effector cells isolated from IL-2 treated, M. leprae -induced PBMC, different responses were observed in leprosy patients and controls. IL-2 stimulated both CD4 and CD8 cytotoxicity of N, and CD8 cytotoxicity of MB, above basal values. In PB patients, the addition of IL-2 tended to enhance the cytotoxic activity of both subpopulations, however, statistical significance was not reached, probably because of the low number of patients (Table 3).
The addition of IFN-γ produced a significant enhancement of cytotoxicity in both CD4 and CD8 effector cells from controls and MB patients.
A stimulatory effect of IL-6 was observed on CD4 and CDS cytotoxicity of normal controls, while in MB patients an enhancement of cytotoxicity was only detected in CD4 effector cells (Table 2). Although IL-6 induced an increase of CD4 and CD8 cytotoxicity in 3 out of 6 PB patients, no significant differences were observed when compared to M. leprae '-basal cytotoxicity (Table 3).
In MB patients, the addition of IL-2, IFN-γ of IL-6 produced a slight enhancement of the lytic activity with respect to untreated effector cells in almost every individual studied. Hence these increments were statistically significant.
Effect of combined cytokines. When IL-6 and IFN-γ were added simultaneously during the induction stage, a significant enhancement of CD4 and CD8 cytotoxicity was observed in MB patients and controls; in PB patients only CDS cytotoxicity was increased (Table 3).
The addition of IL-6 plus IL-2 to MB and control cultures produced a significant enhancement of CD4 and CDS cytotoxicity (Table 3). This combination of cytokines resulted in a CD4- and CD8-mediated cytotoxic activity of MB effector cells similar to the basal values for the controls. Although a positive effect was observed in PB patients, statistical significance was not reached, probably because of the low number of cases.
As shown in Table 3, the addition of IL-6 plus IFN-γ produced a synergistic effect on CD8 from PB patients (p < 0.05), while this effect was only observed in CD4 and CD8 effector cells from MB patients (p < 0.05) by the addition of IL-6 plus IL-2.
DISCUSSION
It is well known that CD4 as well as CDS T cells are required for protection, but their relative contribution depends on the pathogen involved. By infecting nude mice with M. leprae, Maw, el al. (9) have sh own that both CD4 and CDS T cells are important for resistance to M. leprae infection. An essential role for MHC-class II-dcpendent immune mechanisms has been demonstrated in several mycobacterial infections (10, 15). Functional CD8 T cells are also required for an effective protection, as has been demonstrated in MHC-Class I deficient mice (10, 15) in consistency with previous reports that described in vitro cytotoxic CD8 T-cell activation by mycobacteria (13, 31, 34). In a previous work, by blocking assays, we have suggested that in leprosy patients and normal individuals both cytotoxic CD4 and CD8 T cells are generated in vitro by M. leprae (34).
In this report, we demonstrate that after 7 days of PBMC culture without any exogenously added growth factor M. leprae- induced CD4 cells from MB patients were more active than CD8 cells in cytotoxic assays; in PB patients, CD8-mediated cytotoxic activity tended to be higher than that of CD4. Kaleab, et al. (13) suggested that a particulate antigen would favor the generation of CDS cytotoxic T cells, but in our experimental system a particulate antigen, such as M. leprae, induced both CD4 and CD8 cytotoxic T lymphocytes (CTL). The contribution of these T-cell subpopulations to the overall cytotoxicity was different in the three groups studied. Although CD4 T cells are prominent in tuberculoid granulomas (7), and induction of CD4 CTL by mycobacteria is a general feature (11, 13, 22, 23, 27), cells expressing serine esterase mRNA, a marker of CTL, were more abundant in tuberculoid leprosy lesions and correlated in number with the cytotoxic CD8+ 9.3+ (CD28+) cells (7). Consistent with those results, the contribution of CD8 effector cells to the cytotoxic activity was higher than that of CD4 in PB patients in our experimental system. In controls, the cytotoxic activity of CD4 cells averaged 27% and that of CD8 cells, 15%. This may be due to crossreaclivity of BCG with M. leprae since control individuals were all BCG-vaccinated (34).
On the other hand, we have previously shown that IL-2, IFN-γ and IL-4 modified the cytotoxic activity of PBMC from MB and PB patients (9). In order to determine on which lymphocyte subset these cytokines exert their influence, we analyzed their impact on the generation of effector CD4 and CD8 CTL.
The role of IL-4 in mediating susceptibility to infections via suppression of cell mediated immunity (CMI) has been widely demonstrated (l2, 29); mRNA coding for IL-4 has been detected in lepromatous leprosy lesions, and CD8 suppressor clones that secrete IL-4 have been obtained from leprosy patients (33, 40). On the other hand, in M. lep rae-stimulated PBMC from leprosy patients a heterogeneous pattern of cytokine secretion has been observed in T cells (2" In our system, the presence of IL-4 abolished or inhibited the generation of CD4 effector cells in MB and PB patients and controls. The development of CDS effector cells from MB patients and controls was completely abrogated, while in PB patients this inhibitory effect was less evident. Although IL-4 has been described as a differentiation factor of CTL activity, our results are in accordance with those of Parronchi, et al. (2S ) who studied the generation of CD4 human PPD-specilic T-cell lines or clones. In their system, the addition of IL-4 produced a depressive effect on cytolytic activity (:s).
Moreover, CD4 and CDS T cells lost their cytotoxic function by downregulation of IFN-γ(s "'). So, in our case, the effect of IL4 in PB patients could be attenuated by the production of IFN-γ, as has been observed in lesions (•''') and in M. /eprae-stimulated (PBMC) (:s).
The presence of IL-2 during the induction of CTL enhanced CDS cytotoxicity in MB patients. IL-2 plays an essential role in the induction of CDS CTL differentiation 8), as was demonstrated in IL-2 "knockout" mice by the lack of differentiation of CTL precursors into effector cells (3S). It has been demonstrated that growth and differentiation of CDS T cells depends on IL-2 secreted by CD4 T cells or exogenously added IL-2 (:). It is well known that MB patients fail to produce IL-2 ("') upon M. lep rae-stimulus. Therefore, the addition of this cytokine to the cell cultures could induce the activation of CDS precursor cytotoxic cells. In PB patients, although IL-2 enhanced CD4 and CDS cytotoxicity, more patients should be studied in an effort to attain statistical significance.
There is growing evidence that IFN-γis central to protective immunity against mycobacterial infections by activating the bactericidal activity of macrophages. IFN-γis released by T cells (l7) and modulates proliferative as well as effector T-cell responses (6, 39) . In our system, the addition of IFN-γ enhanced the cytotoxic activity of both CD4 and CD8 lymphocyte subpopulations obtained from MB patients and normal controls. Likewise, it has been shown that the addition of IFN-γ to bulk cultures favored the development of CD4 T cells into clones that possess cytolytic activity (28).
Several groups have shown recently that IL-6 can induce CTL development (25, 30), and its role in mycobacterial infections is not clear. IL-6 induced CD4 cells to produce IL-2 and IL-4 which are the mediators for final differentiation of CD8 CTL (30). The enhancement of CD4 cytotoxicity from controls and MB patients, and of CD8 cytotoxic activity from controls by the addition of IL-6, may be explained by the induction of IL-2 secretion from CD4 T cells, or CD8 precursors that produce and use IL-2 (3). Although, IL-6 had only a slight effect on CD4 cytotoxicity in MB patients, the simultaneous addition of IL-2 and IL-6 to MB cells produced a synergistic effect on both CD4 and CD8 effector cells, resulting in the highest values of cytotoxic activity. IL-6 has been observed in lesions (41) as well as in PBMC from leprosy patients (16). IL-6, with other type 2 cytokines, would be expected to promote antibody production and suppress T-cell responses (37, 42). Quite the opposite, our results show that IL-6 acts as an important signal in the development of M. leprae- induced cytotoxicity. Thus, although in ENL lesions a type 2 cytokine pattern is observed, an increase in IL-6 secretion and the presence of IFN-γ and IL-2 (7,41) would favor the induction of CTL activity, resulting in the clearance of macrophages harboring mycobacteria.
On the other hand, type 1 cytokines like IFN-γ have been detected in tuberculoid lesions (40). Here, we demonstrate that in PB patients, CD8 cytotoxicity was significantly enhanced by IL-6 plus IFN-γ. So one may assume that the presence of these cytokines could contribute to enhance MHC-class I restricted cytotoxicity. It has been demonstrated that CD8 T cells might interact in vivo with Schwann cells (36); hence the presence of a CD8-mediated cytotoxic activity in PB patients could explain the nerve damage observed in these patients and in reactional states.
Our results therefore suggest that IL-6 in combination with IFN-γ or IL-2 would be important for the generation and/or modulation of CTL in leprosy patients. Furthermore, the resultant M. leprae- specific CTL activity could be responsible for the protection as well as the tissue damage in M. leprae infection.
Acknowledgment. This work was supported by grants from the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR), from CONICET, and from the Fundaciôn "Alberto J. Roemmers," Argentina. We gratefully acknowledge Fundación de la Hemofilia for the use of the FACScan cytometer, and Dr. Norma Riera, Dr. Nora Galassi and Miss Marta Felippo for skillful technical assistance with flow cytometry. We are also indebted to Dr. María Marta de E. de Bracco for helpful discussions and revision of the manuscript.
REFERENCES
1. BARNES, P. F, MISTRY, S. D., COOPER, C. L., PIRMEZ, C, REA. T. H. and MODLIN, R. L. Compartmentalization of a CD4-sup+ T lymphocyte subpopulation in tuberculous pleuritis. J. Immunol. 142(1989)1114-1119.
2. BASS, H. Z., YAMASHITA, N. and CLEMENT, L. Heterogeneous mechanisms of human cytotoxic T lymphocyte generation. 1. Differential helper cell requirement for the generation of cytotoxic effector cells from CD8+ precursor subpopulations. J. Immunol. 149(1992)2489-2495.
3. BASS, H. Z., YAMASHITA, N. and CLEMENT, L. Heterogeneous mechanisms of human cytotoxic T lymphocyte generation. II. Differential effects of IL-6 on the helper cell-independent generation of CTL from CD8+ precursor subpopulations. J. Immunol. 151(1993)2895-2903.
4. BOOM, W. H., WALLIS, R. and CHERVENAK. K. A. Human Mycobacterium tuberculosis- reactive CD4+ T cell clones: heterogeneity in antigen recognition, cytokine production, and cytotoxicity for mononuclear phagocytes. Infect. Immun. 59(1991)2737-2743.
5. BOYUM, A. Isolation of mononuclear cells and granulocytes from human blood. Scand. J. Clin. Lab. Invest. 21 Suppl. 97(1968)77-89.
6. CHEN, L., TOURVIELLE, B., BURNS, G. F, BACH, F. M., MATHIEU MAHUL. D., SASPORTES, M. and BEN-SUSSAN, A. IFN: a cytotoxic T lymphocyte differentiation signal. Eur. J. Immunol 16(1986)767-770.
7. COOPER, C. L., MUELLER, C, SINCHAISRI, T. A., PIRMEZ, C, CHAN, J., KAPLAN, G., YOUNG, S., WEISSMAN, S. M. M., BLOOM, B. R., REA, T. and MODLIN, R. L. Analysis of naturally occurring delayed type hypersensitivity reactions in leprosy by in situ hybridization. J. Exp. Med. 169(1989)1565-1581.
8. ERARD, F., WILD, M. T., GARCIA SANZ, J. A. and LE GROS, G. Switch of CD8 T cells to noncytolytic CD8 CD4 cells that make Th2 cytokines and help B cells. Science 260(1993)1802-1805.
9. FINK, S., DE LA BARRERA, S., MINNUCCI, F., VALDEZ, R., BAI.IÑA, L. M. and SASIAIN, M. C. IFN-γ, IL-6 and IL-4 modulate M. leprae- or PPD-specific cytotoxic T cells in leprosy patients. Scand. J. Immunol. 38(1993)551-558.
10. FLYNN, J. L., GOLDSTEIN, M. M.. TRIEBOLD, K., KOLLER, B. and BLOOM, B. Major histocompatibility complex class I-restricted T cells are required for resistance to Mycobacterium tuberculosis infection. Proc. Natl. Acad. Sci. U.S.A. 89(1992)12013-12017.
11. HAANEN, J. B. A., DE; WAAL MALEFIJT, R., RES, P. C. M., KRAAKMAN, E. M., OTTENHOFF, T. H. M., DE VRIES, R. R. and SPITS, H. Selection of a human T helper type-1 like T cell subset by mycobacteria. J. Exp. Med. 174(1991)583-592.
12. HEINZEL, F. P.. SADICK, M. D., MUTHA. S. S. and LOCKSLEY, R. M. Production of interferon gamma. interleukin-2, interleukin-4 and interleukin-10 by CD4+ lymphocytes in viva during healing and progressive murine leishmaniasis. Proc. Natl. Acad. Sci. U.S.A. 88(1991)7011-7015.
13. KALEAB, B., OTENHOFF, CONVERSE, P., HALAPI, E., TADESSE, G., ROTTEMBERG, M. and KIESSLING, R. Mycobacterial-induced cytotoxic T cells as well as non-specific killer cells derived from healthy individuals and leprosy patients. Kur. J. Immunol. 20(1990)2651-2659.
14. KUMARARATNE, D. S., PlTHIE, A. S., DRYSDALE, P., GASTON, J. S. H., KIESSLING, R., ILES, P. B., ELLIS, C. J., INNES, J. and WISE, R. Specific lysis of mycobacterial antigen-bearing macrophages by class II MHC-restricted polyclonal cell lines in healthy donors or patients with tuberculosis. Clin. Exp. Immunol. 80(1990)314-323.
15. LADEL, C, DAUGELAT, S. and KAUFMANN, S. H. E. Immune response to Mycobacterium bovis BCG infection in major histocompatibility complex class I- and II-delicient knock out mice: contribution of CD4 and CD8 T cells to acquired resistance. Eur. J. Immunol. 25(1995)377-384.
16. LAUNOIS, P., VANDENBUSSCHE, P., N'DIAYE: NIANG, M., DROWART, A., VAN VOOREN, J. P., SARTHOU, J. L., MILLAN, J. and HUYGEN, K. IL-6 production in response to purified mycobacterial heat-shock proteins and to antigen 85 in leprosy. Cell Immunol 148(1993)283-290.
17. MACKANESS, G. B. The influence of immunologically commited lymphoid cells on macrophage activity in vivo. J. Exp. Med 129(1969)973-992.
18. MAKAKOVSKI, E., CHEN, W. F. and SHORTMAN, K. IL-2 and IFNγ are two necessary lymphokines in the development of cytolytic T cells. J. Immunol. 143(1989) 1210-1214.
19. MAW, W. W., TOMIOKA, H., SATO, K., YAMADA, Y. and SAITO, H. Study of the roles of CD4+ and CD8+ T cells in the expression of host induced in athymic nude mice. Int. J. Lepr. 63(1995)539 545.
20. MISRA, N., MURTAZA, A., WALKER, B., NARAYAN, N. P. S., MISRA, R. S., RAMESH, V., SINGH, S., COLSTON, M. J. and NATH, I. Cytokine profile of circulating T cells of leprosy patients reflects both indiscriminate and polarized T-helper subsets: T-helper phenotype is stable and influenced by related antigens of Mycobacterium leprae. Immonology 86(1995)97-103.
21. MODLIN, R. L., KAPLAN, J. M., YOUNG, S., PIRMEZ, C, KINO, H., CONVIT, J., REA, T. H. and BLOOM, B. R. Learning from lesions: pattern of tissue inflammation in leprosy. Proc. Natl. Acad. Sci. U.S.A. 85(1988)1213-1217.
22. MUSTAFA, A. S. and GODAL, T. BCG-induced CD4+ cytotoxic T cells from BCG vaccinated healthy subjects: relation between cytotoxicity and suppression in vitro. Clin. Exp. Immunol. 69(1987)255-262.
23. MUTIS, T, CORNELISSE, Y. E. and OTTENHOFF, T. H. M. Mycobacteria induce CD4+ T cells that are cytotoxical and display Th1 -like cytokine secretion profile: heterogeneity in cytotoxic activity and cytokine secretion levels. Eur. J. Immunol. 23(1993)2189-2195.
24. MUTIS, T., KKAAKMAN, E. M., CORNELISSE, Y. E., HAANEN, J. B., SPITS, H., DE VRIES, R. R. P. and OTTENHOFF, T. H. M. Analysis of cytokine production by Mycobacterium- reactive T cells; failure to explain M. leprae -specific nonresponsiveness of peripheral blood T cells from lepromatous leprosy patients. J. Immunol. 151(1993)4641-4651.
25. OKADA, M., KITAHARA, M., KISHIMOTO, S., MATSUDA.T, HIRANO. T. and KISHIMOTO T. IL-6/BSF-2 functions as a killer helper factor in the in vitro induction of cytotoxic T cells. J. Immunol. 141(1988)1543-1549.
26. OKADA, M., KLIMPEL, G. K., KUPPERS, R. C. and HENNEY, C. S. The differentiation of cytotoxic T cells in vitro. 1. Amplifying factor(s) in the primary response is Lytl cell dependent. J. Immunol. 122(1979)2527-2533.
27. OTTENHOFF, T. H. M., KALEAB, B., VAN EMBDEN,J. D. A., THOLE J. E. R. and KIESSLING, R. The recombinant 65 kD beat shock protein of Mycobacterium bovis BCG/ M. tuberculosis is a larget molecule for CD4+ cytotoxic T cell lymphocytes that lyse human monocytes. J. Exp. Med. 168(1988)1947-1952.
28. PARRONCHI, P., DE CARLI, M., MANETTI, R., SIMONELLI, C, SAMPOGNARO, S., PICCINI, M. P., MACCHIA, D., MAGGI, E., DEL PRETE, G. and ROMAGNANI, S. IL-4 and IFN (a and γ) exert opposite regulatory effects on the development of cytolytic potential by Th1 or Th2 T cell clones. J. Immunol. 149(1992)2977-2983.
29. PEARCE, E. J., CASPAR, P., GRYCH, J. M., LEWIS, F.A. and SHER, A. Downregulation of Th1 cytokine production accompanies induction of Th2 responses by a parasitic helminth, Schistosoma mansoni. J. Exp. Med. 173(1991)159-166.
30. QUENTMEIER, H., KLAUCHE, J., MUHLRADT, P. and DREXLER, H. Role of IL-6, IL-2 and IL-4 in the in vitro induction of cytotoxic T cells. J. Immunol. 149(1992)3316-3320.
31. REES, A. D., SCOGING, A., MEHLERT, A., YOUNG, D. B. and IVANYI, J. Specificity of proliferative response of human CD8 clones to mycobacterial antigens. Eur. J. Immunol. 18(1988)1881-1887.
32. RIDLEY, D. A. and JOPLING, W. H. Classification of leprosy according to immunity; a live-group system. Int. J. Lepr. 34(1966)255-273.
33. SALGAME, P., AURAMS, J. S., CLAYBERGER, C, GOLDSTEIN, H., CONVIT, J., MODLIN, R. L. and BLOOM, B. Differing lymphokine profiles of functional subsets of human CD4 and CD8 T cell clones. Science 254(1991)279-282.
34. SASIAIN, M. DEL C, DE LA BARRERA, S., MINNUCCI, F, VALDEZ, R., DE E. DE BRACCO, M. M. and BALIÑA, L. M. T-cell mediated cytotoxicity against Mycobacterium antigen pulsed autologous macrophages in leprosy patients. Infect. Immtin. 60(1992)3389-3395.
35. SCHORTE, H., HOLRSCHKE, T., HONIG, T., SCHIMPL, A. and HOKAK, I. The development and function of T cells in mice rendered IL-2 deficient by gene targeting. Nature 352(1991)621-624.
36. STEINHOFF, U. and KAUFMANN, S. H. E. Specific lysis by CD8 + T cells of Schwann cells expresing M. leprae antigens. Eur. J. Immunol. 18(1988)969-972.
37. ULICH T. R., YIN, S., GUO, K., YI, E. S., REMICK, O. and DEL CASTILLO, J. Intratracheal injection of endotoxin and cytokines. IL-6 and TNF β inhibit acute inflammation. Am. J. Pathol. 138(1991)1097-1101.
38. VAN VOOKHIS, W. C, KAPLAN, G., SARNO, E. N., HORWITZ, M. A., STEINMAN, R. M., LEVIS, W. R., NOOVEIRA, N., HAIR, L. S., GATTAS, C. R., ARRICK, B. A. and REA, T. H. The cutaneous infiltrates of leprosy; cellular characteristics and the predominant phenotypes. N. Engl. J. Med. 307(1982)1593-1597.
39. VOLC-PLATZER, B., STEMBERG, H., LUGER, T., RADASZKiEWICZ, T. and WIEDERMANN, G. Defective intralesional interferon-gamma activity in patients with lepromatous leprosy. Clin. Exp. Im-munol. 71(1988)235-240.
40. YAMAMURA, M., UYEMURA, K., DEANS, J. R.,WEINBERG, K., REA, T. H., BLOOM, B. R. and MODLIN, R. L. Defining protective responses to pathogens: cytokine profiles in leprosy lesions.Science 254(1991)277-279.
41. YANIAMURA, M., WANG, X. H., OHMEN, J. D., UYEMURA, K., REA, T. H., BLOOM, B. R. and MODLIN,R. L. Cytokine patterns of immunologically mediated tissue damage. J. Immunol. 149(1992)1470-1475.
42. ZHOU, D. H., MUNSTER, A. and WINCHURCH, R. D.Pathologic concentrations of IL-6 inhibit T cell responses via induction of activation of TCG-β. FASEB J. 5(1991)2582-2585.
1. Ph.D., Departamento Inmunología, Instituto de Investigaciones Hematológicas, Academia Nacional de Medicina, Pacheco de Lemo 3081, 1425 Buenos Aires, Argentina.
2. Ph.D., Departamento Inmunología, Instituto de Investigaciones Hematológicas, Academia Nacional de Medicina, Pacheco de Lemo 3081, 1425 Buenos Aires, Argentina.
3. Ph.D., Departamento Inmunología, Instituto de Investigaciones Hematológicas, Academia Nacional de Medicina, Pacheco de Lemo 3081, 1425 Buenos Aires, Argentina.
4. M.D., Ph.D., Dermatologia, Hospital "Gral San Martin," UBA, Buenos Aires, Argentina.
5. M.D., Facultad de Medicina, Universidad de Rosario, Buenos Aires, Argentina.
6. M.D., Ph.D., Buenos Aires, Argentina.
7. Ph.D., Departamento Inmunología, Instituto de Investigaciones Hematológicas, Academia Nacional de Medicina, Pacheco de Lemo 3081, 1425 Buenos Aires, Argentina.
Reprint requests to Dr. Maria del C. Sasiain at the above address or FAX 54-1-803-9475.
Received tor publication on 22 August 1996.
Accepted tor publication in revised form on IS November 1996.