- Volume 65 , Number 1
- Page: 56–62
Immunotherapy of lepromin-negative borderline leprosy patients with low-dose convit vaccine as an adjunct to multidrug therapy; a six-year follow-up study in Calcutta
ABSTRACT
The present report, which describes management of lepromin-negative borderline leprosy patients with low-dose Convit vaccine, is an extension of our earlier study on the treatment of lepromatous leprosy patients with low-dose Convit vaccine as an adjunct to multidrug therapy (MDT). The test Group I, consisting of 50 lepromin-negative, borderline leprosy patients, were given low-dose Convit vaccine plus MDT. The control group II consisted of 25 lepromin-negative, borderline leprosy patients given BCG vaccination plus MDT and 25 lepromin-negative, borderline leprosy patients given killed Mycobacterium leprae (human) vaccine plus MDT. The control group III consisted of 50 lepromin-positive, borderline leprosy patients not given any immunostimulation but given only MDT. Depending upon the lepromin unresponsiveness, the patients were given one to four inoculations of the various antileprosy vaccines and were followed up every 3 months for 2 years for clinical, bacteriological and immunological outcome. All patients belonging to the test and control groups showed clinical cure and bacteriological negativity within 2 years. However, immunologic potentiation, assessed by lepromin testing and the leukocyte migration inhibition test (LMIT), was better in the test patients receiving low-dose Convit vaccine plus MDT than in the control patients receiving BCG vaccine plus MDT or killed M. leprae vaccine plus MDT or MDT alone. But the capacity of clearance bacteria (CCB) test f rom the lepromin granuloma showed poor bacterial clearance in the test patients. However, there was no relapse during 6 years of follow up. Two mid-borderline (BB) patients had severe reversal reactions with lagophthalmos and wrist drop during immunotherapy despite being given low-dose Convit vaccine.RÉSUMÉ
Le présent rapport, qui décrit la prise en charge de patients borderline négatifs à la lépromine avec une faible dose du vaccin de Convit, est une extension de notre étude précédente sur le traitement de patients lépromateux avec une faible dose du vaccin de Convit en appui à la polychimiothérapie (PCT). Le groupe test I, consistant en 50 patients borderline négatifs à la lépromine. a reçu une faible dose du vaccin de Convit plus la PCT. Le groupe témoin II consistait en 25 patients borderline négatifs à la lépromine ayant reçu le vaccin BCG et 25 patients borderline négatifs à la lépromine avant reçu le vaccin de Mycobacterium leprae tué (d'origine humaine) plus la PCT. Le groupe témoin III consistait en 50 patients borderline positifs à la lépromine qui n'ont reçu aucun immunostimulant, mais bien la PCT. En fonction de leur non-réponse à la lépromine, les patients ont reçu de une à quatre injections des différents vaccins antilèpre et ont été suivis du point de vue clinique, bactériologique et imnumologique tous les trois mois pendant deux ans. Tous les patients appartenant aux groupes test et témoins ont montré une guérison clinique et bactériologique endéans les deux ans. Cependant, la potentialisation immunologique, évaluée par le test à la lépromine et le test d'inhibition migratoire des leucocytes (TIML), était meilleure chez les patients ayant reçu une faible dose du vaccin de Convit plus la PCT que chez les témoins ayant reçu le BCG plus la PCT ou le vaccin de M. leprae tué plus la l'CT ou la l'CT seule. Mais le test de capacité d'élimination bactérienne à partir du gandióme de lépromine a montré une faible élimination chez, les patients du groupe test. Cependant, il n'y a pas eu de rechute au cours des six ans de follow-up. Deux patients borderline (BB) ont eu des réactions d'inversion sévères avec lagophlalmos et poignet tombant durant l'immunothérapie, en dépit du lait qu'ils avaient reçu une faible dose du vaccin de Convit.RESUMEN
El presente reporte describe el manejo de pacientes con lepra intermedia lepromino-negativos, con una dosis baja de la vacuna de Convity es una extensión de nuestro primer estudio sobre el tratamiento de pacientes con lepra lepromatosa con vacuna de Convity poliquimioterapia (PQT). til grupo de prueba I (50 pacientes con lepra intermedia lepromino-negativos) recibió una dosis baja de vacuna de Convit más PQT. El grupo control II consistió de 25 pacientes con lepra intermedia lepromino-negativos. que recibieron la vacuna BCG más la PQT, y de 25 pacientes con lepra intermedia y lepromino-negativos que recibieron la vacuna de Mycobacterium leprae (humano) muerto por calor además de la PQT. El grupo control III consistió de 50 pacientes con lepra intermedia lepromino-positivos que no recibieron vacuna alguna pero sí la PQT. Dependiendo de la reactividad a la lepromina, los pacientes recibieron de tina a 4 inoculaciones de las diferentes vacunas antileprosas y fueron seguidos cada 3 meses durante 2 años para establecer los cambios clínicos, bacteriológicos e inmunológicos. Todos los pacientes de los grupos de prueba y control mostraron curación clínica y negatividad bacteriológica dentro del periodo de 2 años. Sin embargo, la potenciación innuinológica. establecida por la prueba de la lepromina y la prueba de inhibición de la migración de linfocitos. fue mejor en los pacientes que recibieron la vacuna de Convit de dosis baja más PQT que en los pacientes control que recibieron la vacuna de BCG más PQT, o la vacuna de M. leprae muerto más PQT, o sólo la PQT. La capacidad de depuración de bacterias de los granulomas producidos por la lepromina fue pobre en todos los grupos estudiados pero no se observaron recaídas en los 6 años de seguimiento. Dos pacientes con lepra intermedia (BB) tuvieron reacciones reversas severas con lagoftalmos y caída de muñeca durante el tratamiento, no obstante que recibieron la vacuna de Convit de dosis baja.Recently, we reported from Calcutta a promising and safe method for the treatment of lepromin-negative, advanced lepromatous leprosy (LL) patients with low-dose Convit vaccine [1.6 x 107 killed Mycobacterium leprae (human) and 1.5 x 105 M. bovis BCG] (10) as an adjunct to the standard multidrug therapy (MDT) in place of the Convit vaccine containing 6.4 x 108 killed M. leprae (armadillo) supplemented by 0.1 mg BCG used in the Venezuelan trial (7), They were given one to six inoculations of the mixed vaccines at 3-month intervals until lepromin conversion. Thereafter, the patients were followed up for a period of 2 years for clinical, bacteriological and immunological outcome. Within 18 months of starting chemo-immunotherapy all patients showed remarkable clinical improvement and bacterial negativity but, even after six inoculations of the mixed vaccine, 33% of the patients failed to show lepromin conversion and 50% of the patients remained negative in the leukocyte migration inhibition (LMI) test using M. leprae sonicate. During the course of the vaccine therapy, no severe reversal reaction (RR) or nerve damage was encountered in any patient. On the contrary, the LL patients treated with MDT alone showed delayed bacterial clearance, prolonged clinical recovery, only 5% lepromin conversion, and none of them became LMI positive (10).
Being encouraged by the above results with the low-dose Convit vaccine, in the present study we extended our investigations on the same immunotherapy for the treatment of all types of lepromin-negative, borderline leprosy patients. Moreover, despite the success of the MDT regimen of the World Health Organization (WHO) in the treatment of leprosy patients, the occurrence of some degree of inflammatory reactions (types 1 and 2) in 50% of the borderline leprosy patients still remains a problem (11). In view of the fear of severe RR and nerve damage that may occur in borderline leprosy patients during immunostimulation with vaccine therapy and in the light of their much lower bacterial load and less severe lepromin anergy than LL patients, the number of inoculations of the low-dose Convit vaccine was restricted to four instead of six as were given to our LL patients.
MATERIALS AND METHODS
One-hundred-fifty, untreated borderline leprosy patients (87 males and 63 females) were taken from the Outpatient Department, School of Tropical Medicine, Calcutta. Their mean age was 37.8 years; mean duration of illness, 36.6 months. The diagnosis was based on clinical, bacteriological and histological findings as well as lepromin testing with 0.1 ml standard lepromin containing 1.6 x 107 killed M. leprae (human). The criteria of lepromin reactivity was based on that described by Harboe (9). A slit-skin smear test was done for all patients at the beginning and at the end of the study (3). Of all the 150 borderline leprosy patients, 100 were Mitsuda negative and their mean bacterial index (BI) was 2.45+. The remaining 50 patients were Mitsuda positive (spontaneous) with a mean BI of 1.05+. The patients were histologically classified on the Ridley and Jopling scale (8).
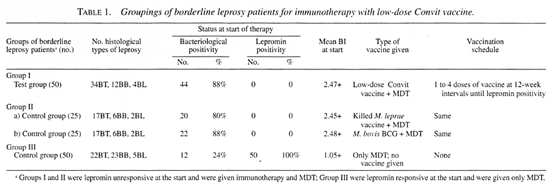
Grouping of patients. All of the 150 borderline patients were divided into three well-matched groups: Group I (test) included 50 lepromin-negative patients, and they were given a mixed vaccine plus MDT. Group II (lepromin-negative controls) consisted of 50 lepromin-negative patients. They were subdivided into two groups: a) 25 patients were given killed M. leprae (human) vaccine plus MDT; b) the other 25 patients were given BCG vaccine plus MDT. Group III (lepromin-positive controls) included 50 Mitsuda-positive (spontaneous) patients who required no immunostimulation and were treated with only MDT (Table 1)(5).
Vaccines. Three types of vaccines were used: a) Convit vaccine: Each dose had 1.6x107 heat-killed M. leprae (human) supplemented by 1.5 x 105 M. hovis BCG (Japan) suspended in 0.1 ml saline and mixed before injection. Human M. leprae were obtained from human lepromas routinely processed in our laboratory. The preparation of the vaccine has been described elsewhere (5). b) Killed M. leprae (human) vaccine: Each dose contained 1.6 x 107 heat killed M. leprae (human) in 0.1 ml saline, c) M. bovis BCG vaccine: Each dose had 1.5 x 105 BCG (Japan) in 0.1 ml saline.
Before the administration of each inoculation, all of the patients were tested for lepromin reactivity with standard lepromin (human) (routinely made in our laboratory) (10). Only those patients remaining lepromin negative after inoculation received subsequent inoculations. The interval between the two successive inoculations was about 12 weeks. Depending on the severity of lepromin anergy, the number of vaccinations given to the test and control patients varied from one to four. The schedule of vaccinations is shown in Table 1.
Clinical outcome. After starting chemoimmunotherapy all of the patients were followed at 3-month intervals for a period of 2 years to study their clinical course, any RR and other clinical complications.
Bacteriological negativity. The bacterial index (BI) of each patient was recorded at the start and at the end of the study by employing the standard technique (3).
Immunological testing. All of the patients were tested twice (once at the beginning and again at the end of treatment) to study any augmentation of specific cell-mediated immunity against M. leprae (human) antigen following chemo-immunotherapy. Three methods were employed: a) the in vivo standard lepromin test (described earlier); b) the capacity of clearing bacteria (CCB) test. This in-vivo test was performed at the end of the study in all patients. The method was described by Convit, et al. (6) . In brief, a massive dose of autoclaved human M. leprae (6.4 x 107 in 0.1 ml saline) was injected intradermally. Biopsies of the injected sites were taken 6 weeks after injection and histopathological studies were done; c) the in-vitro leukocyte migration inhibition (LMI) test against sonicated M. leprae (human) antigen was done for all patients at the start and at the end of the treatment. The method has been described else where (1).
RESULTS AND DISCUSSION
An inter-laboratory study of armadilloderived M. leprae vaccine revealed striking differences between the preparations of M. leprae provided by the WHO for use in leprosy vaccine trials (13). We used human-derived M. leprae vaccine which obviated those differences.
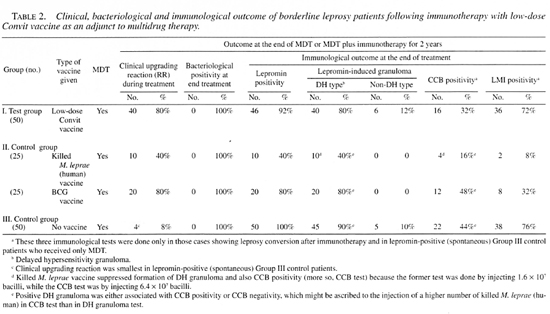
Table 2 shows the clinical, bacteriological and immunological outcome of the borderline leprosy patients following administration of the three types of currently popular antileprosy vaccines, all patients belonging to the test and control groups showed clinical cure and bacteriological negativity within the 2-year study period. However, 80% of the test patients, immunostimulated by low-dose Convit vaccine (Group I), and another 80% of the control patients, receiving BCG vaccination (Group IIb), had undergone a clinical upgrading reaction and had shown a histological shift of polarity toward the tuberculoid end. Clinically upgrading (reversal) reactions were manifested by exacerbation of existing lesions, neuritis of already involved nerves, the appearance of new lesions and involvement of new nerves within 6 weeks of starting immunotherapy. On the contrary, only 40% of the control patients receiving immunologic stimulation with the killed M. leprae (human) vaccine (Group IIa) had undergone a clinical upgrading reaction and had shown a shift of histological polarity (Table 2). Fifty spontaneously lepromin-responsive, control borderline leprosy patients (Group III), who had a low mean BI (1.05+) (Table 1), received no antileprosy vaccine. Curiously, only 40% of these patients showed a clini cal upgrading reaction (Table 2). The reaction was so severe in two mid-borderline (BB) leprosy patients receiving low-dose Convit vaccine therapy (Group I) that one developed facial paralysis with lagophthalmos and another developed bilateral foot and wrist drop which necessitated steroid therapy. This perhaps diluted out the gain of immunostimulation offered by the vaccine therapy. However, up to now there is no suitable method to predict such disaster before starting immunotherapy. These results, thus, show that vaccine-driven, leprominreactive, borderline leprosy patients (Group I) can show better clinical and histological reversal reactions than the spontaneously lepromin-positive, borderline leprosy patients with a low bacterial load (Group III), who were not given any vaccine therapy. Also, low-dose Convit vaccine was more effective than the killed M. leprae (human) vaccine in terms of clinical and histological reversal reactions. This needs explanation: Perhaps killed M. leprae (human) in the mixed vaccine could stimulate M. leprae -specilic T-helper cells, vis-a-vis, it could also activate M. leprae -specific T-suppressor cells (12), while BCG in the mixed vaccine could, in addition, activate M. leprae- loaded macrophages and eliminate intracellular bacteria.
Although all of the borderline leprosy patients in both test and control groups became bacteriologically negative within the study period, the lepromin-conversion rates were variable (Table 2). Thus 8% of the borderline leprosy patients receiving Convit vaccine, 20% of the patients receiving BCG vaccine, and 60% of the patients administered killed M. leprae vaccine remained refractory to lepromin challenge, despite receiving four inoculations of the different vaccines. The present results show that our mixed vaccine, although it contained a low quantum of antigen, was a superior immunologic potentiator than BCG or killed M. leprae vaccines. This can be explained by the findings of Chatterjee and her associates (4). These authors reported that lipoarabinomannan (LAM), which is a constituent of M. leprae and BCG, induces secretion of tumor necrosis factor alpha (TNF-α), a cytokine critical for the host defense against mycobacteria and formation of lepromin granuloma, which is the hallmark of protective immunity against leprosy (4).
The results of the in-vitro LMI tests were parallel to that of the in-vivo lepromin tests (Table 2). The LMI test was positive in 72% of the patients receiving low-dose Convit vaccine (Group 1), in 32% of the patients receiving BCG vaccine (Group IIa), and in only 8% of the patients receiving killed M. leprae vaccine (Group IIb). Interestingly, the LMI test was positive in 76% of the spontaneously lepromin-positive, borderline leprosy patients (Group III) who had received no immunotherapy. Nevertheless, all of the lepromin-responsive patients were not LMI positive, showing thereby that a positive lepromin test signified a delayed hypersensitive granulomatous reaction indicative of TNF-α activity (J), while a positive LMI test pointed to the ability of M. leprae -specific T cells to release the lymphokine migration inhibition factor (MIF) after being challenged with sonicated M. leprae antigen in vitro.
The CCB (capability of clearance of bacteria) tests in our patients, on the other hand, were not parallel to the lepromin and LMI tests (Table 2). A positive lepromin test in a tuberculoid patient is a yardstick of immunologic stability and finds the histopathologic concurrence in the formation of specific delayed hypersensitive granuloma composed of epithelioid cells, lymphocytes and giant cells in and around neurovascular complexes in the dermis. Thus, their illness should resolve spontaneously. But, in practice, the disease remains active in about 50% of the tuberculoid leprosy patients even after a specified period of MDT. In fact, all Group II patients were spontaneously lepromin-positive but they still had borderline leprosy. In order to investigate this enigma, we looked into the functional aspects of the macrophages within the lepromin granuloma formed after injecting a large amount (6.4 x 107) of heat-killed M. leprae (human) in our patients at the end of the study period, and we looked for the ability of the patients to eliminate the intracellular mycobacteria from the lepromin granuloma. Interestingly, the CCB positivity rates were very low in comparison to the lepromin positivity rates (Table 2). It was in only 32% of the patients receiving low-dose Convit vaccine (Group I), in 48% of the patients receiving BCG vaccination (Group IIa), in 16% of the patients administered killed M. leprae (human) vaccine (Group IIb), and in 44% of the spontaneously lepromin-positive borderline patients receiving only MDT (Group III). Why was the CCB test positivity rate (32%) in the patients receiving Convit vaccine (Group I) lower than that (48%) of the patients receiving the BCG vaccine (Group IIa)? Phenolic glycolipid-I (PGL-I) and LAM in M. leprae, a constituent of the Convit vaccine, perhaps depressed the macrophage function, such as O; production, and were unable to kill and eliminate M. leprae from the macrophages :). A strong immune response could be mounted by a better second-generation subunit vaccine containing only immunodominant epitopes (Mukerjee, R. Vaccines for leprosy-present status and future prospects. Erwin Stindl Memorial Oration. Calcutta: Greater Calcutta Leprosy Treatment and Health Education Scheme, 1989, pp. 9-13), so that it might eliminate the persistors and prevent relapse or reactivate the disease processes. During the present 6year follow-up study, no relapse occurred in any of the patients belonging to both the test and control groups.
Finally, the authors wish to compare the results of the treatment of lepromin-negative LL patients (mean BI 3.49+) with one to six injections of low-dose Convit vaccine plus MDT (1) with the result of the present study on the treatment of lepromin-negative borderline leprosy (mean BI 2.47+) patients with one to four inoculations of vaccines of the same potency. The lepromin and LMI conversion rates were less in the LL patients (66% and 50%, respectively) (1) than those in the borderline leprosy patients (92% and 72%, respectively) (present study). These comparative results thus indicate severe immunologic anergy in the LL patients in comparison to the borderline patients. Severe reversal reaction with nerve paralysis occurred in two borderline leprosy patients (present study) despite the fact that they were given only four inoculations of low-dose Convit vaccine. On the contrary, the reversal reactions occurring in two LL patients and ENL in one LL patient following immunostimulation with one to six injections of low-dose Convit vaccine were mild and required no steroid therapy.
REFERENCES
1. BLOOM, B. R. and GHADE, P. R. In-vitro Methods in Cell-Mediated Immunity. New York: Academic Press, 1971.
2. BRITTON , W. J. Leprosy 1962-1992: immunology of leprosy. Trans. R. Soc. Trop. Med. Hyg. 87(1993)508-514.
3. BRYCESON, A. and PFALTZGRAFF, R. E. Leprosy. 2nd edn. Edinburgh: Churchill Livingstone, 1979, pp. 28-64.
4. CHATTERJEE, D., MCNEIL, M. and BRENNAN, P. J. Structural features of arabinan components of lipoarabinomannan of Mycobacterium tuberculosis. J. Biol. Chem. 226( 1991)9562-9660.
5. CHAUDHURY, S., HAJRA, S. K., SAhA, B., MAZUMDER, B., CHATTOPADHYA, D. and SAHA, K. An eight-year field trial on antileprosy vaccines among high-risk household contacts in the Calcutta metropolis. Int. J.Lepr. 62(1994)389-394.
6. CONVIT, J., AVILA, J. L., GOIHMEN, M. and PlNARDI, M. E. A test for the determination of competency in clearing bacilli in leprosy patients. Bull. WHO 46(1972)821-826.
7. CONVIT, J., ARANZAZU, N., UI.KKTI, M., PINARDI, M. E., ROYES, O. and ALVARADO, J. Immunotherapy with a mixture of Mycobacterium leprae and BCG in different forms of leprosy and in Mitsuda negative contacts. Int. J. Lepr. 50(1982)415-424.
8. DHARMENDRA . Classification of leprosy. In: Leprosy. Hastings, R. C, ed. Edinburgh: Churchill Livingstone, 1989, pp. 88-99.
9. HARBOE, M. The immunology of leprosy. In: Leprosy. Hastings, R. C, ed. Edinburgh: Churchill Livingstone, 1989, pp. 53-87.
10. MAJUMDER, V., MUKERJEE, A., HAJRA, S. K., SAHA, B. and SAHA, K. Immunotherapy of far-advanced patients of lepromalous leprosy with lowdose Convit vaccine along with multidrug therapy (Calcutta trial). Int. J. Lepr. 61(1996)26-36.
11. PFALTZGRAFF, R. E. and BRYCESON, A. Clinical leprosy. In: Leprosy. Hastings, R. C, ed. Edinburgh: Churchill Livingstone, 1985, pp. 134-186.
12. RADA, E., CONVIT, J., ULRICH, M., GALLINOTO, M. E. and ARANZAZU, N. Immunosuppression and cellular immunity reaction in leprosy patients treated with a mixture of Mycobacterium leprae and BCG. Int. J. Lepr. 55(1987)646-650.
13. WORLD HEALTH ORGANIZATION. Immunology of Mycobacterial Diseases (IMMYC) Steering Committee. Analysis of vaccines prepared from armadillo derived M. leprae; results of an interlaboratory study coordinated by the World Health Organization. Int. J. Lepr. 63(1995)48-55.
14. WHO LEPROSY UNIT. Risk of relapse in leprosy. Indian J. Lepr. 67(1995)13-26.
15. WHO STUDY GROUP. Chemotherapy of leprosy for control programmes. Geneva: World Health Organization, 1982. Tech. Rep. Ser. 675.
1. M.B.B.S., D.C.O., Ph.D., Professor of Leprology (deceased);
2. M.B.B.S., D.T.M.&H., D.C.P., Ph.D., Reader of Leprology (retired);
3. M.D., D.C.P., Ph.D., Professor of Pathology and Director (retired);
4. M.B.B.S., D.T.M.&H., M.D., Assistant Professor of Leprology;
5. M.B.B.S., D.V.D., Demonstrator of Leprology, School of Tropical Medicine. Calcutta, India.
6. M.D., Joint Director, National Institute of Communicable Diseases, Delhi, India.
7. M.Sc, M.B.B.S., Ph.D. (USA), Professor of Immunology (retired), Delhi University, Delhi, India.
Reprint requests to Dr. K. Saha. 45A Sova Bazar Street, Calcutta 700 006, India.
Received for publication on 16 July 1996.
Accepted for publication in revised form on 11 November 1996.