- Volume 65 , Number 1
- Page: 63–72
Dharmendra antigen but not integral M. leprae is an efficient inducer of immunostimulant cytokine production by human monocytes, and M. leprae lipids inhibit the cytokine production
ABSTRACT
Killed integral Mycobacterium leprae, Mitsuda antigen, and chloroform-treated M. leprae, Dharmendra antigen (Dh-Ag), have been used for the classification of leprosy patients based on cell-mediated immunity. Heat-killed M. leprae also are used as a component of the Convit vaccine. Human blood monocytes were stimulated with M. leprae or Dh-Ag and their cytokine-inducing ability was compared. Monocytes were cultured in the presence of fresh human serum because the efficacy of cytokine induction and the phagocytosis of M. leprae have been shown to be optimal in the presence of fresh serum. M. leprae and Dh-Ag were equally phagocytosed by monocytes. Dh-Ag was more potent than M. leprae in the induction of immunostimulatory/proinflammatory cytokines, interleukin-1 (IL-1), IL-6 and tumor necrosis factor (TNF). In contrast, a comparable level of IL-1ra, an immunosuppressive cytokine, was induced by M. leprae and Dh-Ag. The lipids extracted f rom M. leprae induced none of these cytokines by monocytes. Nevertheless, when monocytes were pretreated with the lipids followed by stimulation with Dh-Ag, productions of IL-1, IL-6 and TNF were all inhibited in a dose-dependent manner. However, the lipids did not inhibit the cytokine production induced by other stimuli, including BCG and lipopolysaccharide. Moreover the lipids did not affect the production of IL-1ra. These results suggest that the lipids f rom M. leprae are responsible for the poor cytokine-inducing ability of M. leprae, thus favoring their infection. These results also suggest that Dh-Ag rather than integral M. leprae may be useful as a vaccine candidate because Dh-Ag is able to induce a large amount of cytokines f rom monocytes.RÉSUMÉ
Du Mycobacterium leprae entier tué, l'antigène de Mitsuda, et du M. leprae traité au chloroforme, l'antigène de Dharmendra, ont été utilisés pour la classification des malades de la lèpre sur base de leur immunité à médiation cellulaire. Du M. leprae tué par la chaleur est aussi utilisé en tant que composant du vaccin de Convit. Des monocytes sanguins humains ont été stimulés par M. leprae et leur capacité à induire des cytokines a été comparée. Des monocytes ont été cultivés en présence de serum humain frais parce qu'on a montré que l'efficacité de l'induction des cytokines et la phagocytose de M. leprae étaient optimales en présence de serum frais. M. leprae et l'antigène de Dharmendra étaient phagocytés de manière équivalente par les monocytes. L'antigène de Dharmendra était plus puissant que M. leprae pour induire les cytokines d'immunostimulation et d'inflammation, l'interleukin-1 (1L-1), 1L-6 et le facteur nécrotique tumoral (FNT). Par contre, un taux comparable de 1L-Ira, une cytokine immunosuppressive, a été induite par M. leprae et l'antigène de Dharmendra. Les lipides extraits de M. leprae n'ont induit la production d'aucune de ces cytokines par les monocytes. Néanmoins, quand des monocytes furent pré-traités avec les lipides, puis stimulés avec l'antigène de Dharmendra, les productions d'IL-l, d'lL-6 et FNT furent toutes inhibées avec une intensité qui était fonction de la dose. Cependant, les lipides n'ont pas inhibé la production de cytokine induite par d'autres stimuli, y compris le BCG et le lipopolysaccharide. De plus, les lipides n'ont pas affecté la production d'IL-Ira. Ces résultas suggèrent que les lipides en provenance de M. leprae sont responsables de la faible capacité de M. leprae à induire des cytokines, favorisant ainsi leur infection. Ces résultats suggèrent également que c'est l'antigène de Dharmendra, plutôt que du M. leprae entier, qui pourrait être utile en tant que candidat vaccin, parce que l'antigène de Dharmendra peut induire la production de grandes quantités de cytokines par les monocytes.RESUMEN
Para la clasificación de los pacientes con lepra se han utilizado Mycobacterium leprae integral, antígeno de Mitsuda, y el antígeno de Dharmendra, Ag-Dh (M. leprae tratado con cloroformo). El M. leprae muerto por calor también se ha usado como un componente de la vacuna de Convit. En este estudio, se estimularon monocitos de sangre humana con M. leprae o con Ag-Dh y se comparó la capacidad de estos materiales para inducir la síntesis de citocinas. Los monocitos se cultivaron en presencia de suero humano fresco porque la eficiencia de la inducción de citocinas y la fagocitosis de M. leprae es óptima en presencia de este componente. M. leprae y el Ag-Dh fueron igualmente fagocitados por los monocitos. El Ag-Dh fue más potente que M. leprae en la inducción de las citocinas inmunoestimulatorias/pro-intlamatorias: interleucina-1 (IL1), IL-6 y factor de necrosis tumoral (TNF). En contraste, tanto M. leprae como el Ag-Dh, indujeron la producción de cantidades comparables de IL-Ira, una citocina inmunosupresora. Los lípidos extraídos de M. leprae no indujeron la síntesis de ninguna de estas citocinas por los monocitos. Sin embargo, cuando los monocitos fueron pretratados con los lípidos y luego se estimularon con el Ag-Dh, se encontró una inhibición en su producción de las citocinas 1L-1, 1L-6 y TNF, que fue dependiente de dosis. Los lípidos no inhibieron la producción de citocinas inducida por otros estímulos, incluyendo BCG y lipopolisacárido. Además, los lípidos no afectaron la producción de 1L-Ira. Estos resultados sugieren que los lípidos de M. leprae podrían ser los responsables de la pobre capacidad inductora de citocinas de M. leprae, hecho que favorecería la capacidad infectante del microorganismo. Ya que el Ag-Dh es capaz de inducir la síntesis de una gran cantidad de citocinas por los monocitos, este antígeno podría ser más util que el M. leprae integral como un candidato a vacuna..Leprosy is a disease in which pathology and immunology are inextricably related (11). Recent studies in the mouse model suggest that the immunologic deviation results from the balance between two types of Thelper cells: Thl cells, producing interleukin-2 (IL-2) and gamma interferon (IFN -γ), stimulate cell-mediated immunity (CMI), while Th2 cells, producing IL-4, IL5 and IL-10), stimulate antibody production (l7). The cytokines produced by one population interfere with the action of the other population. It has been suggested that the cytokines from T cells and macrophages, including IFN -γ, IL-4 and IL-12, induce the differentiation of primitive T-helper cells (ThO) into either type of cells (7,26). In humans, it is reported that IL-2 and IFN -γ mRNA, products of Thl cells, were evidently expressed in tuberculoid leprosy lesions while IL-4, IL-5 and ILK) mRNA, products of Th2 cells, were expressed in lepromatous lesions (28).
Macrophages are cells interacting with Mycobacterium leprae in the early phase of infection in which M. leprae are able to grow. Since macrophages produce a variety of cytokines and present antigen to T cells, the primary interaction between macrophages and M. leprae may be pivotal in determining the immunological deviation. IL-1, IL-6 and TNF are the major cytokines produced by macrophages, and these cytokines stimulate immunological and inflammatory reactions (8). In contrast, the IL-1 receptor antagonist (IL-1ra), which also is produced by macrophages, counteracts IL-1 action by competitively binding to the IL-1 receptor (2,9). Therefore, macrophage function is regulated by a balance of these immunostimulatory/proinflammatory and immunosuppressive/antiinflammatory cytokines.
In our previous paper, we studied the ability of M. leprae and M. bovis BCG to induce cytokine production by human monocytes (24) . M. leprae appeared to be a very poor inducer of immunostimulatory cytokines compared to BCG, but they did induce a comparable level of IL-1ra. Based on the results, we suggested that M. leprae may escape the host's defense by evoking little immunostimulatory cytokine production. We also considered the possibility that a strain of M. leprae capable of inducing the production of immunostimulatory cytokines could be obtained, and that this strain might be suitable for vaccination. Killed integral M. leprae have been used for the diagnosis of M. leprae infection as Mitsuda antigen and as a component of the Convit vaccine, a mixture of killed M. leprae and live BCG (5). Dharmendra antigen (Dh-Ag) is free of chloroform-soluble lipids, including specific phenolic glycolipid-I (PGL-I), a potential immunosuppressive component, and has been used for the evaluation of CMI in patients (6).
In this study we have compared the ability of killed integral M. leprae and Dh-Ag in inducing cytokines by human monocytes. The effects of the lipids from M. leprae on cytokine production was also studied.
MATERIALS AND METHODS
Reagents. Human recombinant IL-1α(2 x 107 U/mg) and TNF-α (2 x 106 U/mg) were provided by Dr. M. Yamada of the Dainippon Pharmaceutical Co., Osaka, Japan; human recombinant IL-2 by Shionogi Co., Osaka, Japan; human recombinant IL-6 by Dr. Y. Akiyama of the Ajinomoto Co., Yokohama, Japan. Concentrated buffy coat from healthy donors was supplied by the An Bichi Red Cross Blood Center, Aichi, Japan. RPMI 1640 and polymyxiwere purchased from Sigma Chemical Co., St. Louis, Missouri, U.S.A., and fetal bovine serum (FBS) was purchased from Bocknek, Toronto, Canada.
Mycobacteria. M. leprae, Thai-53 strain, were grown in the foot pads of nude mice (14). The mouse foot pads were aseptically removed, minced with scissors and homogenized with 7H12 medium. After centrifuging the homogenate for 10 min at 100 x g, the supernatants were obtained and again centrifuged for 20 min at 3500 x g. The pellets were resuspended with 7H12 medium and the bacillary number was determined by the method of Shepard and McRae (21). The bacillary number was consistent with that counted under the microscope with a hematocytometer. Freeze-dried M. bovis BCG were obtained from the Japan BCG Company, Tokyo, Japan, and heat-treated at 120șC for 20 min. BCG were suspended with phosphate buffered saline (PBS). These mycobacteria were homogenized by mild sonication. The bacillary number was counted under the microscope with a hematocytometer. Heat-killed M. leprae were obtained by treating them at 120șC for 15 min.
Preparation of Dh-Ag. Dh-Ag was prepared according to the method of Dharmendra (7). M. leprae, Thai 53 strain grown in nude mice, were killed by treating the cells at 120șC for 20 min, and then extracted with 25 volumes of chloroform at 4șC for 3 days. After removing the chloroform by evaporation, the cells were washed with ether. After drying, the pellet was dispersed in an aqueous solution containing 0.45% NaCl and 0.5% phenol. The suspension was again heat treated at 120șC for 15 min, and used as Dh-Ag. The final concentration of phenol was 0.02% at 107 bacilli/ml, and at that dosage the phenol did not affect cytokine production from the monocytes.
Preparation of lipids from M. leprae. The lipids from M. leprae were prepared by the method of Bligh and Dyer (2). M. leprae, Thai 53 strain (7.4 x 109 bacilli) grown in nude mice, were suspended in 1 ml of PBS. To the suspension, 3.75 ml of C:M (chloroform : methanol, 2:1 v/v) solution was added, and then vigorously shaken for 2 min. To the mixture, 1.25 ml of chloroform was added, shaken for 30 min, and then 1.25 ml of PBS was added and again shaken for 30 min. The final mixture was centrifuged at 1 150 x g x 5 min, and then the chloroform layer was obtained. To the residual water layer 2 ml of chloroform was added, and the same extraction procedure was repeated. The chloroform layer thus obtained was combined with the former chloroform layer. The pooled chloroform extracts were evaporated, and the residue was dissolved with the C:M solution to achieve a concentration equivalent to 1010 bacilli/ml, and stored at -20șC until used. A total of 0.027 g of lipids was obtained.
Preparation of monocyte supernatants. The buffy coat from healthy donors was diluted 1:3 in Hank's balanced salt solution (HBSS). The mononuclear cells (MNC) were separated over Ficoll-Hypaque, treated with lysis buffer (0.017 M Tris-HCl, pH 7.65, containing 0.75% NH4C1) to remove contaminant erythrocytes and platelets, washed twice in HBSS, and suspended in RPMI 1640 medium supplemented with 100 U/ml of penicillin G, 100 µ g/ml of streptomycin, and 15 mM HEPES. The number of monocytes was estimated by incubating the cell suspension in a hematocytometer at 37șC for 3 min in air containing 5% CO2 and then counting the spreading cells. The spreading cell number was consistent with that of cells adhering to the tissue culture plate. One ml of the MNC suspension containing monocytes (1 x 106 cells/ml) was added to each well of a 24well plate (Falcon, Lincoln, New Jersey, U.S.A.). After 2 hr of culturing at 37șC in air containing 5% CO2 the cells were washed twice with HBSS and 80% to 90% of the adherent cells were monocytes as determined by morphological criteria with Giemsa staining and the ability to phagocytose latex beads. To the adherent monocytes, 1 ml of RPMI 1640 supplemented with 1% fresh human AB serum containing M. leprae or Dh-Ag was added. The cells were then cultured at 37șC. Prior to the addition of M. leprae and Dh-Ag, the cells were homogenized by a mild treatment with sonication using a well-type sonicator (Bransonic 220) for 1 min. Although the medium was endotoxin-free according to the Limulus amoebocyte assay (sensitivity limit of 0.1 ng/ml), we usually added polymyxin B (5 µ g/ml) to the culture, except for the stimulation with lipopolysaccharide (LPS), to rule out the possible effect of an undetectable level of endotoxin. After each culture period, the supernatants were obtained by centrifugation.
Phagocytosis of monocytes of M. leprae and Dh-Ag and morphological examination. The monocytes (I x 106 cells/ml) were cultured in RPMI 1640 medium supplemented with 1% fresh human AB serum with M. leprae and Dh-Ag on cover slips in 24-well culture plates. After culture for 24 hr, the cover slips were washed and stained with acid-fast reagents. Phagocytosis of the mycobacteria by the monocytes and morphological changes were examined under the microscope.
Assay for IL-1 activity. IL-1 activity was determined by a proliferation assay with an IL-1-dependent mouse T-cell line (D10N4M) which was provided by Dr. S. J. Hopkins of the University of Manchester, U.K. (I2). In brief, cells were cultured in RPMI 1640, 10 mM HEPES, antibiotics, 5 x 10-5 M 2-mercaptoethanol, 10% FBS, Concanavalin-A (ConA; 3 µ g/ml), IL-2 (40 U/ ml) and standard recombinant human IL-1 a or test samples. Cells (1 x 104) were cultured in wells of flat-bottom microtiter plates at 37șC in 5% CO2 in air. After 3 days of culture, cell proliferation activity was assessed by the MTT method (16). After solubilization of formazan with 20% sodium dodecyl sulfate (SDS) and 50% dimethyl formamide in water, the absorbance at 595 run was measured in an ELISA autoreader (Bio-Rad Laboratories, Richmond, California, U.S.A.). IL-1 activity was expressed as the unit equivalent to standard recombinant IL-1 α.
Assay for IL-6 activity. The biological activity of IL-6 was measured by its proliferative action on the IL-6-dependent murine hybridoma clone MH60.BSF2 provided by Dr. T. Hirano of Osaka University, Japan (l3). Proliferation was measured by the MTT method. One unit of IL-6 activity was defined as the reciprocal of the dilution of samples that exhibited 50% of the maximum response.
Assay for TNF activity. The activity of TNF was determined by a L929 fibroblast cell lytic assay (25). Briefly, 100 µ l of a suspension of TNF-sensitive mouse L929 fibroblast cells (5 x 105 cells/ml) was cultured with serially threefold diluted test samples in wells of a flat-bottom microtiter plate at 37șC for 18 hr in air containing 5% CO2 in the presence of actinomycin D (1 µ g/ml). After culture, the plates were washed, and the cell lysis was determined by staining the plates with crystal violet (0.5%) in methanol-water (1:25 v/v). Human recombinant TNF-α was used as a positive control to give 100% lysis. After solubilizing the dye-stained cells with 0.1 ml of 0.1% SDS, the dye uptake was measured by an ELISA autoreader. One unit of TNF activity was defined as the reciprocal of the dilution of samples that lysed 50% of the L929 cells.
Determination of IL-1ra. IL-1ra content was determined by an ELISA using mouse monoclonal antibody (IgG) and rabbit polyclonal antibody (IgG) against recombinant human IL-1ra. Briefly, the samples and standard IL-1ra were added to the wells of 96-well plates precoated with mouse monoclonal IgG against IL-1ra. After washing, the rabbit polyclonal IgG against IL-1ra was added, and then reacted with goat horseradish peroxidase-conjugated anti-rabbit IgG followed by incubation with the enzyme substrate solution (0.4 M citrate-0.2 M sodium phosphate buffer containing 11 mg/ml o -phenylenediamine, 0.01% H2O2). The plates were read at 490 nm by an ELISA autoreader.
RESULTS
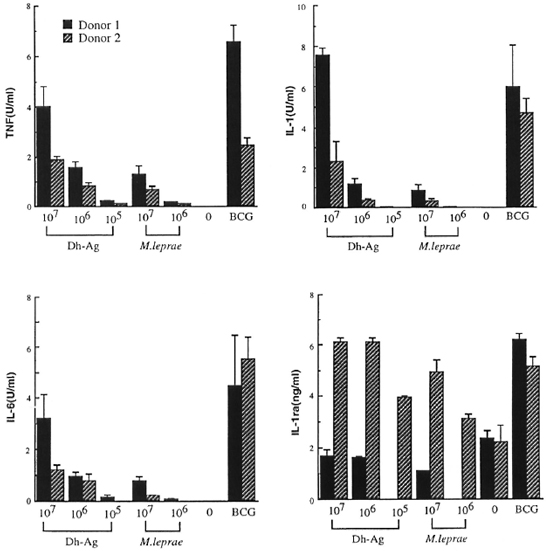
Comparison of cytokine production by monocytes stimulated with M. leprae and Dh-Ag. In order to compare the ability of M. leprae and Dh-Ag in the induction of IL-1, IL-6, TNF and IL-1ra by human monocytes, monocytes (1 x 106 cells) were cultured with varying numbers of heatkilled M. leprae and Dh-Ag. Because a previous study showed that optimal cytokine production by monocytes stimulated with M. leprae or BCG and phagocytosis of the bacilli required the presence of fresh human serum, in this study monocytes were cultured in the presence of 1 % fresh human serum (24). Under the culture conditions, M. leprae and Dh-Ag were equally phagocytosed by monocytes as determined by acidfast staining, and there were no apparent morphological changes in the monocytes (data not shown). Previous kinetic studies showed that 1L-1, 1L-6 and TNF productions were maximum after 24 hr of culture, but IL-1ra production increased with the duration of the culture period. Therefore, the IL-1, IL-6 and TNF contents were determined after 24 hr of culture, and IL-1ra after 72 hr of culture. Killed BCG were used as a positive control because they have been shown to be a very potent inducer of these cytokines. Two representative results of five healthy donors are shown in Figure 1. Unstimulated monocytes produced none of these cytokines. Small amounts of TNF, IL-1 and IL-6 were induced by 107/ml of M. leprae. However, M. leprae at 107ml induced only trace amounts of cytokines. Although the data are not shown, 105ml M. leprae did not induce these cytokines. In contrast, large amounts of TNF, IL-1 and I L-6 were induced by Dh-Ag at either 107 or 106/ml. At 105/ml Dh-Ag still induced these cytokines. The maximum potency of Dh-Ag to induce TNF, IL-1 and IL-6 was almost comparable to that of BCG. In contrast, there was not much difference between M. leprae and Dh-Ag in the production of IL-1ra. Although the data are not shown, all donors exhibited similar responses to these stimuli.
Fig 1. Cytokine production from human peripheral blood monocytes stimulated with M. leprae , Dh-Ag orBCG. Monocytes (1 x 106 cells/ml/well) were cultured for 24 hr or 72 hr with or without killed M. leprae , Dh-Ag (107 to 105/ml/well), or killed BCG (106/ml/well). After culture, concentrations of IL-1, IL-6, TNF and IL-1ra in the culture supernatants were determined. Mean ± S.D. of triplicate cultures is shown.
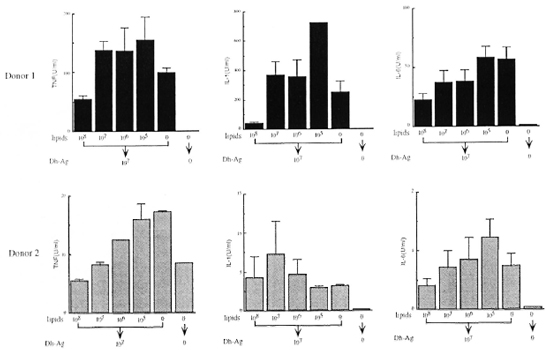
Effect of lipids from M. leprae on cytokine production. We extracted the lipids from M. leprae, and examined whether or not they induced cytokine production by monocytes. However, the lipids at concentrations equivalent to 108 to 106 bacilli/ml induced no detectable TNF, IL-1, IL-6 or IL-1ra (data not shown). In order to determine the effect of the lipids on the cytokinc-inducing ability of Dh-Ag, monocytes were pretreated with the lipids for 6 hr, and then the cells were stimulated with Dh-Ag. As shown in Figure 2, the productions of IL-1, IL-6 and TNF were all inhibited in a dose-dependent manner by the lipid pretreatment. Although in donor 2 1L-1 production was not affected by the lipids, in the other four donors the production was consistently inhibited (data not shown). Lipids at concentrations as high as equivalent to 108 bacilli/ml did not affect the bioassay of these cytokines. In addition, the lipids were not toxic to monocytes as judged by determinations of trypan blue exclusion, cell number, or MTT staining.
Fig. 2. Effect of pretreatment with lipids from M. leprae on cytokine production from monocytes stimulatedwith Dh-Ag. Monocytes (1 x 106 cells/ml/well) were pretreated with varying concentrations of the lipids (equivalent to 108 to 105 bacilli/ml) for 6 hr. Dh-Ag was then added and the cells were cultured for another 24 hr. Concentrations of IL-1, IL-6 and TNF in the culture supernatants were determined. Mean ± S.D. of triplicate culturesis shown.
We then examined the effect of the lipids at a concentration equivalent to 108 bacilli/ ml on the cytokine-inducing ability of other stimuli, BCG and LPS. The effect of the lipids on IL-1ra production was also examined. As shown in Figure 3, although the lipids again inhibited the stimulating effect of Dh-Ag, they did not inhibit the effects of BCG or LPS. The slight inhibitory effect of the lipids on IL-6 production in response to BCG was not observed in any other donor (data not shown). IL-1ra production was not influenced by pretreatment with the lipids. Although we conducted experiments with varying concentrations of BCG and LPS using monocytes of two other donors, again, the lipids were unable to inhibit cytokine production (data not shown).
Fig. 3. Effect of lipids from M. leprae on cytokine production from monocytes stimulated with various stim-uli. Monocytes (I x 106 cells/nil/well) were pretreated with the lipids (equivalent to 107 bacilli/ml) for 6 hr. Dh-Ag (107/ml), BCG (106/ml) or LI'S (100 ng/ml) were added, and the cells were cultured for another 24 hr or 72hr. Concentrations of IL-1, IL-6, TNF and IL-1ra in the culture supernatants were determined. Mean ± S.D. of triplicate cultures is shown. The amount of IL-1ra was determined in the pooled supernatants of triplicate cultures. Data from one of two representative donors with similar results are shown.
DISCUSSION
Dh-Ag has been used for a very long time to evaluate CMI in leprosy patients (7). However, there has been no investigation of its interaction with monocytes. In this study, we demonstrate the marked difference between integral M. leprae and Dh-Ag in regard to their ability to induce cytokine production from human monocytes. Our previous studies using FBS and human serum, either untreated or heat-inactivated, demonstrated that phagocytosis and the cytokine-inducing ability of M. leprae were optimum in the presence of fresh human serum (24). It is reported that complement C3 binds to PGL-I in M. leprae, thereby facilitating phagocytosis of the bacilli by fresh monocytes through the complement receptors CR1 and CR3 (19). Therefore, our findings support this idea. In addition, we consider fresh human scrum more physiologic to use than heat-inactivated human serum or FBS. Thus, in this study the experiments were conducted in the presence of fresh human serum.
It was considered that Dh-Ag may be more refractory to phagocytosis because lipids, including PGL-I, were removed from the bacilli. However, we failed to observe any differences between Dh-Ag and M. leprae with respect to phagocytosis probably because other components are also involved in the phagocytosis. On the other hand, it is possible that components generated by the chloroform treatment may contribute to the phagocytosis. In M. tuberculosis, the terminal mannose of lipoarabinomannan (LAM) is reported to participate in the binding and phagocytosis of the bacilli (20). Since most of M. leprae LAM could not be removed under our experimental conditions, this possibility is likely.
Irrespective of the similarity in phagocytosis between M. leprae and Dh-Ag, there were many differences in cytokine induction. TNF, IL-1 and IL-6 stimulate immune reactions through activation and/or induction of proliferation or differentiation of immune cells, including monocytes/macrophages, T cells, B cells and natural killer cells. These cytokines are known to regulate the function and/or production of each other in both positive and negative manners. In monocytes or macrophages, TNF induces IL-1 and IL-6, and IL-1 induces TNF and IL-6 (x). In contrast, IL-6 is unable to induce IL-1 or TNF, but it instead suppresses the production of IL-1 and TNF (s). As previously shown, M. leprae were very poor inducers of TNF, IL-1 and IL-6. The poor ability of M. leprae was not the result of the heat treatment of the bacilli because live M. leprae also were unable to induce much cytokines from monocytes. In contrast, Dh-Ag appeared to be a potent inducer of these cytokines. Since the levels of induced cytokines by Dh-Ag at 106/ml were the same as those induced by M. leprae at 107/ml, Dh-Ag is about 10-fold more potent than M. leprae. Since the efficiency of phagocytosis was not different, the difference seems to be due to post-phagocytosis events.
In contrast to these cytokines, IL-1ra (an immunosuppressive cytokine) was induced comparably by M. leprae and Dh-Ag. IL-1ra interferes with the binding of IL-1 to its receptors, thus inhibiting the action of IL-1 as well as the cascade reaction induced by IL-1 both in vitro and in vivo (22). Since macrophage function is determin ed by the balance between immunostimulatory and immunosuppressive cytokines, Dh-Ag appeared to be a more efficient immunostimulant than M. leprae. It has been reported that Dh-Ag induced a higher lymphocyte proliferative response than integral M. leprae in both tuberculoid leprosy patients and normal individuals (4 ,21). Our present findings provide a basis for the differences in these two preparations as to their ability to stimulate lymphocyte proliferation.
The differences between whole M. leprae and Dh-Ag are quite interesting. We show that the lipids from M. leprae alone induced none of these cytokines, and that pretreatment of monocytes with the lipids inhibited the production of all of the cytokines, except IL-Ira, from monocytes stimulated with Dh-Ag. However, we were unable to observe the inhibitory effect when monocytes were stimulated with BCG or LPS. Therefore, the inhibitory effect seems to be specific to Dh-Ag. This finding is interesting when considering the specific anergy against M. leprae antigen in lepromatous leprosy patients. The inhibitory effect was not due to a cytotoxic effect of the lipids because the lipid-treated monocytes exhibited no reduction in cell viability, cell number, or MTT staining. Therefore, the lipids appear to be responsible for the poor potency of M. leprae in the induction of cytokines.
M. leprae lipids contain PGL-I and LAM. PGL-I is a strong antibody-generating antigen in patients, although the antibody does not contribute to the host defense against M. leprae (1). Since PGL-I is a major lipid component of M. leprae and is associated with the outer surface of the bacilli, it is thought to work as a virulence factor. In lepromatous patients the serum level of antibody against PGL-I is high and the "foam" seen in heavily infected macrophages characteristic of the lepromatous granuloma is thought to contain much PGL-I. It has been reported that PGL-I inhibited the oxidative response of human monocytes stimulated with M. leprae, but did not affect the stimulating effects of other stimuli, such as PMA, zymosan, BCG or M. kansasii (27). It has also been stated that PGL-I inhibited the production of IL-1, IL-6 and TNF from human monocytes stimulated with LPS (23). Although, in our study, the inhibitory effect of the lipids was specific to Dh-Ag, we must wait for the purification of the suppressive molecule to ascertain its specificity. In any case, it is possible that PGL-I functions as a suppressive factor for the induction of cytokines.
Another candidate for the suppressive entity is LAM. LAM from avirulent, but not virulent, M. tuberculosis induced TNF production by murine macrophages or human monocytes (15,18). LAM from M. leprae not only inhibited T-cell proliferation, it also induced a refractory response to IFN -γ in mouse macrophages to kill or inhibit the multiplication of a subsequent infection with Toxoplasma gondii (21). In addition, LAM from M. leprae did not induce TNF from murine macrophages (1). It is of note, however, that LAM is covalently linked to the peptidoglycan, and that most of the LAM is associated with Dh-Ag. Dh-Ag is still more potent than M. leprae in cytokine induction. Therefore, it is unlikely that LAM from M. leprae is the predominant suppressive molecule for cytokine production in human monocytes.
Cytokines, especially IFN -γ and TNF, alone or in synergy, activate macrophages to kill intracellular mycobacteria (10). Our study, therefore, suggests that the lipids of M. leprae play a role in escaping the macrophagc-killing effect by suppressing the induction of cytokines. Also, the lipids are important to the inhibition of the accumulation and/or activation of granulocytes and macrophages by inhibiting the production of IL-1 and TNF because these cytokines are potent inducers of chemokines.
It is known that vaccination requires an adjuvant which is a potent inducer of cytokines from monocytes or macrophages. Indeed, killed integral M. leprae are used as Convit vaccine in the concomitant presence of live BCG (5,6), a potent inducer of immunostimulant cytokines. Since Dh-Ag is mostly free from PGL-I, and is able to induce much cytokine production from monocytes, Dh-Ag may be suitable for vaccination.
Acknowledgment. The authors wish to thank Dr. M. Matsuoka of the National Institute for Leprosy Research for providing M. leprae grown in the nude mouse and Dr. D. Yang of Nagoya City University for reviewing this manuscript. This study was supported in part by a grant from the U.S.-Japan Cooperative Medical Sciences Program. The authors also wish to acknowledge the Sasakawa Memorial Health Foundation for the financial support of this study.
REFERENCES
1. ADAMS, L. B., FUKUTOMI, Y. and KRAHENBUHL, J. L. Regulation of murine macrophage effector functions by lipoarabinomannan from Mycobacterium strains with different degrees of virulence. Infect. Immun. 61(1993)4173-4181.
2. AREND, W. P., SMITH, M. F, JR., JANSON, R. W. and JOSLIN, F. G. IL-1 receptor antagonist and IL10 production in human monocytes are regulated differently. J. Immunol. 147(1991)1530-1536.
3. BJUNE, G. Comparison of various preparations of Mycobacterium leprae and other mycobacteria by lymphocyte stimulation. Int. J. Lepr. 46(1978)386-393.
4. BLIGH, E. G. and DYER, W. J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37(1959)911-917.
5. CONVIT, J., ARANZAZU, N.. ULRICH, M., PINARDI, M., REYES, O. and AI.VARADO, J. Immunotherapy with a mixture of M. leprae and BCG in different forms of leprosy and in Mitsuda-negative controls. Int. J. Lepr. 50(1982)415-424.
6. CONVIT, J., SAMPSON, C, ZUNIGA, M., SMITH, P. G., PLATA, J., SILVA, J., MOLIANA, J., PINARDI, M. E., BLOOM, B. R. and SALGADO, A. Immunoprophylactic trial with combined Mycobacterium leprae BCG vaccine against leprosy: preliminary results. Lancet 339(1992)446-450.
7. DHARMENDRA, P. The lepromin test; a review. Lepr. Rev. 18(1947)92-126.
8. DlNARELLO, C. A. Interleukin-1 and its biologically related cytokines. Adv. Immunol. 44(1989)153-205.
9. DlNARELLO, C. A. and THOMPSON, R. C. Blocking IL-1: interleukin 1 receptor antagonist in vivo and in vitro. Immunol. Today 12(1991)404-410.
10. FLESCH, I. E., HESS, J. H., OSWALD, I. P. and KALLMANN, S. H. E. Growth inhibition of Mycobacterium bovis by IFN-gamma stimulated macrophages: regulation by endogenous tumor necrosis factor-alpha and by IL-10. Int. Immunol. 6(1994)693-700.
11. HASTINGS, R. C, GILLIS, T. P., KRAHENBUHL, J. L. and FRANZBLAU, S. G. Leprosy. Clin. Microbiol. Rev. 1(1988)330-348.
12. HOPKINS, S. J. and HUMPHREYS, M. Simple, sensitive and specific bioassay for interleukin-1. J. Immunol. Methods 120(1989)271-276.
13. MATSUDA, T, HIRANO, T. and KlSHIMOTO, T. Establishment of an interleukin 6 (IL-6J/B cell stimulatory factor 2-dependent cell line and preparation of anti-IL-6 monoclonal antibodies. Eur. J. Immunol. 18(1988)951-956.
14. MATSUOKA, M., KOHSAKA, K. and DAYANGHIRANG, A. J. Characterization of M. leprae Thai-53 strain. (Abstract) Int. J. Lepr. 60(1992)722.
15. MORENO, C, TAVERNE, J., MEHLERT, A., BATE, C. A. W., BREALEY, R. J., MEAGER, A., ROOK, G. A. W. and PLAYEAIR, J. H. L. Lipoarabinomannan from Mycobacterium tuberculosis induces the production of tumor necrosis factor from human and murine macrophages. Clin. Exp. Immunol. 76(1989)240-245.
16. MOSMANN, T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity. J. Immunol. Methods 65(1983)55-63.
17. MOSMANN, T. R. and MOORE:, K. W. The role of IL-10 in cross regulation of TH1 and TH2 responses. Immunol. Today 12(1991)A49-A53.
18. ROACH, T. I., BARTON, C. H., CHATTERJEE, D. and BLACKWELL, J. M. Macrophage activation; lipoarabinomannan from avirulent and virulent strains of Mycobacterium tuberculosis differentially induces the early genes c-fos, KC, JE, and tumor necrosis factor-α. J. Immunol. 150(1993)18861896.
19. SCHLESINGER, L. S. and HORWITZ, M. A. Phenolic glycolipid-1 of Mycobacterium leprae binds complement C3 in serum and mediates phagocytosis by human monocytes. J. Exp. Med. 174(1991)1031-1038.
20. SCHLESINGER, L. S., HULL, S. R. and KAUFMAN, T. M. Binding of the terminal mannosyl units of lipoarabinomannan from a virulent strain of Mycobacterium tuberculosis to human macrophages. J. Immunol. 152(1994)4070-4079.
21. SHANNON, E. J., POWELL, M. D., KIRCHHEIMER, W. F. and HASTINGS, R. C. Effects of Mycobacterium leprae antigens on the in vitro responsiveness of mononuclear cells from armadillos to concanavalin-A. Lepr. Rev. 55(1984)19-31.
22. SHEPARD, C. C. and MCRAE, D. H. A method for counting acid-fast bacteria. Int. J. Lepr. 36(1967)78-82.
23. SIBLEY, L. D., HUNTER, S. W., BRENNAN, P. J. and KRAHENBUHL, J. L. Mycobacterial lipoarabinomannan inhibits gamma interferon-mediated activation of macrophages. Infect. Immun. 56(1988)1232-1236.
24. SILVA, C. L., FACCIOLI, L. H. and Foss, N. T. Suppression of human monocyte cytokine release by phenolic glycolipid-I of Mycobacterium leprae. Int. J. Lepr. 61(1993)107-108.
25. SUZUKI, K., FUKUTOMI, Y., MATSUOKA M., TORII, K., HAYASHI, H., TAKII, T, OOMOTO, Y. and ONOZAKI, K. Differential production of interleukin 1 (IL-1), IL-6, tumor necrosis factor, and IL-I receptor antagonist by human monocytes stimulated with Mycobacterium leprae and M. bovis BCG. Int. J. Lepr. 61 (1993) 609-618.
26. TAMATANI, T, KIMURA, S., HASHIMOTO, T. and ONOZAKI, K. Purification of guinea pig tumor necrosis factor (TNF): comparison of its antiproliferative and differentiative activities for myeloid leukemic cell line with those of recombinant human TNF. J. Biochem. 105(1989)55-60.
27. TRINCHERI, G. Interleukin-12 and its role in the generation of TH1 cells. Immunol. Today 14(1993)335-339.
28. VACHULA, M., HOLZER, T. J. and ANDERSEN, B. R. Suppression of monocyte oxidative response by phenolic glycolipid I of Mycobacterium leprae. J. Immunol. 142(1989) 1696-1701.
29. YAMAMURA, M., UYEMURA, K., DEANS, R. J., WEINBERG, K., REA, T. H., BLOOM, B. R. and MODLIN, R. L. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science 254(1991)277-279.
1. M.Sc, Department of Hygienic Chemistry, Faculty of Pharmaceutical Sciences, Nagoya City University, Mizuho, Nagoya 467, Japan.
2. Ph.D., Senior Investigator; National Institute for Leprosy Research, Aoba-cho, Higashimurayama -shi, Tokyo 189, Japan.
3. Ph.D., Laboratory Chief of Laboratory 4, National Institute for Leprosy Research, Aoba-cho, Higashimurayama -shi, Tokyo 189, Japan.
4. Ph.D., Research Scientist, Department of Immunology, Otsuka Pharmaceutical Co., Ltd., Tokushima 770, Japan.
5. B.S., Department of Hygienic Chemistry, Faculty of Pharmaceutical Sciences, Nagoya City University, Mizuho, Nagoya 467, Japan.
6. Ph.D., Associate Professor; Department of Hygienic Chemistry, Faculty of Pharmaceutical Sciences, Nagoya City University, Mizuho, Nagoya 467, Japan.
7. Ph.D., Professor, Department of Hygienic Chemistry, Faculty of Pharmaceutical Sciences, Nagoya City University, Mizuho, Nagoya 467, Japan.
Reprint requests to Dr. Onozaki.
Received lor publication on 25 March 1996.
Accepted for publication in revised form on 7 August 1996.