- Volume 65 , Number 1
- Page: 73–9
Effect of phorbol myristate acetate (PMA) and lonophore A23187 on lnterleukin-2 levels and proliferation of activated T lymphocytes f rom patients with lepromatous leprosy
ABSTRACT
The immunodeficiency present in patients with lepromatous leprosy is characterized by the limited proliferation of T lymphocytes, and is explained in part by the impaired synthesis of interleukin-2 (IL-2). Diacylglycerol (DAG) and calcium produce the activation of PKC, ERK and JNK kinases, implying a normal IL-2 response. Phorbol esters, such as PMA, can substitute for DAG and are mitogenic to human T and B cells activating several cytokine-encoding genes. lonophore A23187 increases calcium permeability across the cellular membrane to the cytosol of lymphoid cells and is considered a co-mitogen of T lymphocytes.Here we report that: 1) PHA-activated T lymphocytes f rom LL patients can be separated into two groups: a) responders (R) with a stimulation index (SI) of > 10 and b) nonresponders (NR) with a SI of < 10. 2) The proliferative responses of cells f rom LL(R), LL(NR) and normal subjects were measured after being stimulated with: I, PHA, PMA, PMA+I PHA+PMA and PHA+PMA+ionophore (PPI). The most important result occurs in LL(NR) patients whose cells did not respond to PHA stimulation but increased to normal levels of proliferation when they were stimulated with PMA. Furthermore, the three groups (NR, R and normals) strongly increased their responses when they were incubated with PPI. 3) Finally, IL-2 concentrations in the supernatants of cultures of T lymphocytes f rom LL(NR), LL(R) and controls were relatively low when they were incubated with PHA or PMA, but the addition of ionophore to PMA and the combination of PHA+ PMA strongly increased the production of IL-2 in all of them, reaching the optimum IL-2 concentration when PPI is used.
It can be concluded that the use of PMA, analogous to DAG, and ionophore A23187 (calcium increaser) in cultures of mitogenactivated T lymphocytes f rom LL patients induced the expression of the IL-2 gene, thus correcting the inadéquate prolifération of T cells f rom LL patients.
RÉSUMÉ
L'immunodélicience présente chez, les patients ayant une lèpre lépromateuse est caractérisée par la prolifération limitée des lymphocytes T. et s'explique en partie par l'altération de la synthèse d'interleukine2 (IL-2). Le diacylglycerol (DAG) et le calcium produisent l'activation des kinases PKC, ERK et JNK. cequi suppose une réponse d'IL-2 normale. Des esters de phorbol, tels que le PMA, peuvent être substitués au DAG et sont mitogènes pour les cellules humaines T et B, activant divers gènes encodant des cytokines. L'inophore A23187 accroît la perméabilité du calcium à travers la membrane cellulaire au cytosol des cellules lymphoides, et est considéré comme un co-mitogène des lymphocytes T.Nous rapportons ici que: 1) Les lymphocytes T activés par le PHA et provenant de patients LL peuvent être séparés en deux groupes: a) les répondants (R), avec un index de stimulation (IS) > 10 et b) les nonrépondants (NR) avec un IS < 10. 2). Les réponses prolifératives de cellules de patients LL(R), LL(NR) et de sujets normaux ont été mesurées après stimulation avec I, PHA, PMA, PMA-1, PHA-PMA et PHA+ PMA+ionophore. Le résultat le plus important survient chez les patients LL(NR) dont les cellules ne répondaient pas à la stimulation avec PHA mais augmentaient à des niveaux normaux de prolifération quand elles étaient stimulées par le PMA. De plus, les trois groupes R, RR et normaux ont fortement augmenté leurs réponses quand ils ont été incubés avec PHA+PMA+ionophore (PPI). 3) Finalement, les concentrations d'IL-2 dans le supernatant des cultures de lymphocytes T en provenance de LL(NR), LL(R) et témoins étaient relativement basses quand elles étaient incubées avec PHA ou PMA, mais l'addition d'ionophore au PMA et les combinaisons de PHA+PMA ont fortement augmenté la production d'IL-2 dans tous les cas, atteignant la concentration optimale d'IL-2 quand on utilisait le PPI.
On peut conclure que l'utilisation du PMA, analogue du DAG et l'ionophore A23187 (accroisseur du calcium) dans les cultures de lymphocytes T activés par de mitogènes provenant de patients LL, comprenait l'expression du gène de 1'IL-2, corrigeant donc la prolifération inadéquate de cellules T provenant de patients LL.
RESUMEN
La inmunodeficiencia presente en los pacientes con lepra lepromatosa se caracteriza por la limitada proliferación de los linfocitos T, y se explica en parte por la síntesis disminuida de interleucina-2 (IL-2). El diacilglicerol (DAG) y el calcio producen la activación de las cinasas PKC, ERK y JNK, implicando una respuesta normal de IL-2. Los esteres forbólicos, como el PMA, pueden substituir al DAG y son mitogénicos para las células T y B humanas, donde actúan activando varios genes codificantes de citocinas. El ionóforo A23 187 (I) aumenta la penetración del calcio al citosol de las células linfodes y se considera como comitógeno de los linfocitos T. En este trabajo nosotros reportamos: ( 1 ) Que los linfocitos T (de pacientes LL) activados con PHA pueden separarse en dos grupos: (a) respondedores (R), con índices de estimulación (IE) de > 10 y (b) norespondedores (NR), con IE de < 10. (2) Que se midieron las respuestas proliferativas de las células (de LLR, LLNR y sujetos normales) estimuladas con I, PHA, PMA, PMA+I, PHA+PMA y PHA+PMA+ionóforo. Aquí, los resultados más importantes ocurrieron en los pacientes LLNR cuyas células no respondieron a la estimulación con PHA pero incrementaron su respuesta proliferativa a valores normales cuando se estimularon con PMA. Además, los 3 grupos. NR, R y normales, aumentaron marcadamente sus respuestas proliferativas cuando se incubaron con PHA+PMA+ionóforo (PPI). (3) Que Las concentraciones de IL-2 en los sobrenadantes de los cultivos de los linfocitos T de LLNR. LLR y controles, fueron relativamente bajas cuando las células se incubaron con PHA o con PMA, pero la adición del ionóforo al PMA y las combinaciones de PHA+PMA aumentaron fuertemente la producción IL-2 en todos ellos. La producción óptima de IL-2 se logró con la combinación PPI.Puede concluirse que el uso de PMA (análogo del DAG) y del ionóforo A23 187 (incrementador de calcio) en los cultivos de linfocitos T (LL) activados con mitógeno, estimula la expresión del gene para IL-2, y corrige la proliferación inadecuada de las células T de los pacientes con lepra.
Lepromatous leprosy is characterized, among other peculiarities, by an antispecific anergy of the cellular immune response (10). This immunodeficiency consists of the limited proliferation of T lymphocytes, and is explained in part by the impaired synthesis of interleukin-2 (IL-2) (12,19). In normal resting T cells, antigens or mitogens induce their proliferation by means of the synthesis of IL-2 and its receptor; the participation of diacylglycerol (DAG) and calcium, that produce the activation of protein kinase C (PKC) (17,23), is mandatory. Phorbol esters such as PMA can substitute for DAG and are autogenic to human T and B cells, producing membrane alterations, modulation of cell-surface receptors (2,5,7,11,18) and activation of several cytokine-encoding genes (3 ,5 , l3, 15).
Ionophore A23187 increases calcium permeability across the cellular membrane into the cytosol of lymphoid cells, and is considered by several authors as a co-mitogen of T lymphocytes (1,16,24). We report here the use of PMA, an analog of DAG and ionophore A23187 (calcium increaser), in cultures of mitogen-activated T lymphocytes from lepromatous leprosy patients in order to induce the expression of the IL-2 gene, thus correcting the inadequate proliferation of T cells from these lepromatous patients.
MATERIALS AND METHODS
Study subjects. Twenty-two patients, 11 males and 11 females between the ages of 20 and 65 years, from the Instituto Dermatológico at Guadalajara, Jalisco, Mexico, were diagnosed as having polar lepromatous leprosy (LL) according to international criteria (21). All presented positive bacilloscopy and all received an irregular treatment of 100 mg of dapsone (diaminodiphenyl sulfone) per day. The length of treatment ranged from 2 to 15 years. The control group consisted of 20 normal subjects, all matched with the study group for sex and age as much as possible.
Mononuclear cells. Heparinized blood (20 IU/ml) was obtained from each subject by venipuncture. After the blood had been centrifuged on a Ficoll-Hypaque gradient (6) at 400 x g , the mononuclear cells were recovered and washed three times with Hanks' balanced salt solution (HBSS). The cells were then suspended in RPMI 1640 culture medium (GIBCO, Grand Island. New York, U.S.A.) supplemented with 5% heat inactivated fetal calf serum (FCS), 2 mM glutamine, 1 mM sodium pyruvate, 0.1 mM nonessential amino acids, 10 mM HEPES, 5 x 10-5 M 2-mercaptoethanol, and penicillin 100 U/ml and streptomycin 100 µ g/ml. Finally, the cells were adjusted to 1 x 106 cells/ml (12).
Proliferation assay. In order to activate T cells from these LL patients, 10 µ g/ml of phytohemagglutinin (PHA) was added to 2 x 105 mononuclear cells, and/or 10 ng/ml of phorbol myristatc acetate (PMA), and/or 1 µ g of ionophore A23187 to the corresponding well, i.e., all possible combinations among the three reagents. The cultures were incubated for 48 hr at 37ºC in a mixture of 95% air and 5% CO2. Thereafter, they were pulsed with 1 µ Ci of 3H-thymidine (specilic activity 6.7 Ci/ µ mole; New England Nuclear, Boston, Massachusetts, U.S.A.).
After a 24-hr incubation, the cells were harvested, and the incorporation of 3Hthymidine was measured in a Packard beta counter. The results were expressed as a stimulation index (SI) according to the following (19):
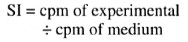
IL -2 assay. The Quantikine Human IL-2 Immunoassay (R and D Systems, Minneapolis, Minnesota, U.S.A.) was used for the quantitative determination of human IL2 concentrations present in the culture supernatants after 48 hr (20).
RESULTS
The Table presents the results of experiments measuring the T-cell proliferation by means of 3H-thymidine incorporation (SI) in PHA-stimulated cell cultures. It can be observed that PHA-activated T lymphocytes from LL patients can be separated into two groups: 1) 9 responders (R) with a SI of > 10 and 13 nonresponders (NR) with a SI of < 10.
Figure 1 shows the proliferative responses of cells from LL(R), LL(NR), and normal subjects stimulated with: I, PHA, PMA, PMA+I, PHA+PMA and PHA+ PMA+ionophore (PPI). It can be seen that ionophore A23187 alone does not cause proliferation. The most important result occurs in LL(NR) patients since their cells that do not respond to PHA stimulation alone increase their proliferation to normal levels when they are stimulated with PHA in the presence of PMA. When lymphocytes from LL(NR) patients are stimulated with PMA+I, they again are less responsive than the LL(R) patients and normal controls. On the other hand, all three groups (NR, R and normals), strongly increased their responses when they were incubated with PPI.
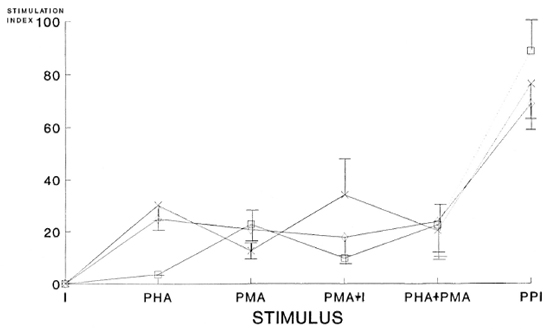
Fig. 1. Proliferative responses (stimulation index) of cells from LL patients [responders (R) and nonresponders (NR)] and normal subjects stimulated with ionophore A23187 (I), PHA, PMA, PMA+I, PHA+PMA, and PHA+PMA+ionophore (PPI). The significance levels of the differences in the SI in each of the following com-binations used in the cultures of cells from LL(R), LL (NR) and normal subjects were: for PHA, p < 0.001 (NRvs each of the other two groups); for PMA, p < 0.004 (R vs each of the other two groups); for PMA+I, p < 0.03(R vs each of the other two groups); for PHA+PMA, p < 0.3 (no differences among groups); for PPI, p < 0.001(NR vs each of the other groups). 1 = Nonresponder (NR); x = responders (R); p = normal subjects.
The significance levels of the differences in each of the following combinations used in the cell cultures from LL(R), LL(NR) and normal subjects were: for PHA, p < 0.001 (NR vs each of the other two groups); for PMA, p < 0.004 (R vs each of the other two groups); when PMA+I was used, p < 0.03 (R vs each of the other two groups); PHA+PMA, p < 0.3 (no differences among groups) and, finally, PPI, p < 0.001 (NR vs each of the other groups).
In Figure 2, the IL-2 concentration in the supernatants of cultures of T lymphocytes from LL(NR), LL(R) and controls stimulated with I, PHA, PMA, PMA+I, PHA+ PMA and PPI are shown. It can be seen that cells from LL(NR) and LL(R) patients and normals do not produce detectable IL-2 levels in the presence of ionophore A23187 alone, and they produce relatively low levels of IL-2 when they are incubated with PHA or PMA. Nevertheless, the combination of ionophore and PMA causes the cells from LL(NR) patients and normals to increase their IL-2 levels. When the PHA+ PMA combination is used, cells from all three groups produce their highest levels of IL-2. Finally, when ionophore A23187 is added to PHA+PMA there is a slight decrease in the production of IL-2 in all of these groups. The significance level of the differences of IL-2 from supernatants of normal cells versus both groups of patients was p < 0.03 when PMA was used; the difference between IL-2 of cells from LL(R) patients versus the other two groups had a significance level of p < 0.04 when PMA+I is used and, finally, the difference between IL-2 of cells from LL(NR) patients versus the other two groups was p < 0.03 when PPI was used.
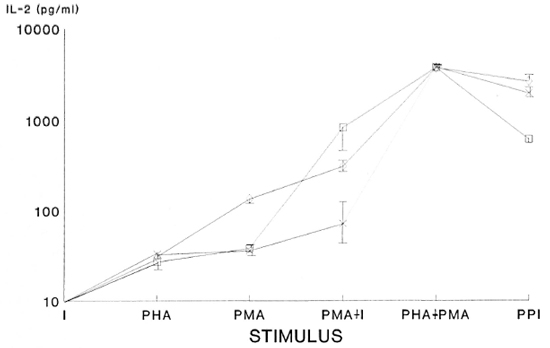
Fig. 2. Interleukin 2 (IL-2) levels found by ELISA in supernatants of cultures of lymphocytes stimulated with ionophore A23I87 (I), PHA, PMA, PMA+1, PHA+PMA and PHA+PMA+ionophore (PPI). The significance levels of the differences of IL-2 concentrations detected by ELISA from supernatants of cells of normalsvs both groups of patients was p < 0.03 when PMA was used; the difference between IL-2 of cells from LL(R) patients vs the other two groups had a significance level of p < 0.04 when PMA+I was used; the difference be-tween IL-2 of cells from LL(NR) patients vs the other two groups had a p < 0.03 when PPI was used. 1 = non-responder (NR); x = responder (R); p = normal subjects.
DISCUSSION
The immunodeficient behavior of T lymphocytes in LL patients has been characterized by a limited proliferation of T cells and a decreased activity of IL-2(9,12,19) Because IL-2 production is, in part, dependent on the intracellular calcium levels and the generation of DAG to activate PKC, we decided to assay the effect of substances that increase cytosolic calcium levels, such as ionophore A23187, and/or substances that mimic DAG such as PMA alone or combined with PHA and ionophore. Our results indicate different kinds of lymphocyte responses from the different groups studied to the above-mentioned stimuli. The first difference is related to the response to PHA of lymphocytes from LL(NR) patients against the LL(R) and normal subjects. The remarkable point is that about 95% of LL(NR) patients become responders when their T cells are stimulated with PMA; the other 5% required the presence of PHA to respond (in the PHA+PMA combination as well as in PPI). When ionophore A23187 is added to PMA their SI decreases, but when PHA+PMA is used they again recover their SI. Conversely, cells from LL(R) patients respond in a mirror image related to the LL(NR) group when PMA+I is used.
All of the groups (normals, responders, nonresponders) reach their highest SI when the triple combination (PPI) is used, implying that PHA, PMA, and ionophore A23187 can trigger enough transduction signals and Ca2+ levels to have "the optimum conditions" for expressing the IL-2 gene, producing the adequate concentration of IL-2.
The explanation for the above-mentioned data is that in the case of cells from LL(NR) patients, PMA is activating PKC, p21 ras (guanosine triphosphate-binding protein), ERK 1 and 2 (extracellular signalregulated protein kinases), RAF (protein kinase, member of the cascade upstream ERK), and MEK (protein kinase, member of the cascade upstream ERK) that had not been activated by PHA alone, implying the expression of the fos gene (8,l4). In the case of LL(R) patients, ionophore A23187 permits enough Ca2+ levels to activate the JNK (c-jun NH2 terminal kinase) system in order to activate the jun gene (s l4). In these experiments, the necessary additions have been done in order that both pathways, in the case of LL(NR) patients and in the LL (R) patients, recover the production of AP-1 (regulatory protein, composed of fos and jun proteins) and, finally, the expression of the IL-2 gene (8,14,22) in order to progress from the Gl phase of the cell cycle to the S phase, G2 and mitosis. This is supported by the fact that IL-2 levels are very high when cells from LL(NR), and LL(R) patients are cultured in the presence of PHA+PMA. The reason why the combination PPI induces the highest SI but not the highest IL-2 levels could be attributed to the fact that IL-2 levels have an optimum dose response effect. An excess of IL-2 does not necessarily imply the highest proliferation index because IL-2 receptors may be shedding from the plasma membrane, thus neutralizing their effect.
In order to corroborate the above explanations, we have initiated experiments that will measure the PKC, ERK, JNK activities (8,12,14).
REFERENCES
1. AKERMAN, K. E. O. and ANDERSSON, L. C. Direct mitogenic effect of ionophore A23187 on isolated human helper lymphocytes. Eur. J. Immunol. 14(1984)286-288.
2. AKITA, Y., OHNO, S., KONNO, Y, YANO, A. and SUZUKI, K. Expression and properties of two distinct classes of the phorbol ester receptor family, four conventional protein kinase C types, and a novel protein kinase C. J. Biol. Chem. 256(1990)354-362.
3. ALBERTS, B., BRAY, D., LEWIS, J., RAFF, M., ROBERTS, K. and WATSON, J. D. Molecular Biol ogy of the Cell. 2nd edn. New York: Garland Publishing, 1989.
4. BAZZI, M . D. and NELSESTUEN, G. L. Constitutive activity of membrane-inserted protein kinase C. Biochem. Biophys. Res. Comm. 152(1988)336-343.
5. BERRY, N., ASE, K.. KIKKAWA, U., KISHIMOTO, A. and NISHIZUKA, Y. Human T cell activation by phorbol esters and diacylglycerol analogues. J. Immunol. 143(1989)1407-1413.
6. Boyum, A. Isolation of mononuclear cells and granulocytes from human blood. Scan. J. Clin. Lab. Invest. 21 Suppl. 197(1988)7785.
7. CHAUHAN, A., CHAUHAN, V. P. S., DESHMUKH, D. S. and BROCKERHOFF, B. Phosphatidylinositol 4,5-biphosphate competitively inhibits phorbol ester binding to protein kinase C. Biochemistry 28(1989)4952-4956.
8. FIELDS, P. E., GAJEWSKI, T. F. and FITCH F. W. Blocked Ras activation in anergic CD4+ T cells. Science 271(1996)1276-1278.
9. HAREGEWOIN, A., MUSTAFA, A. S., HEILLE, I., WATERS, M. F., LEIKER, D. L. and GODAL, T. Reversal by interleukin-2 of the T-cell unresponsiveness of lepromatous leprosy to Mycobacterium leprae. Immunol. Rev. 80(1984)76-86.
10. HASTINGS, R. C, GILLIS, T. P., KRAHENBUHL, J. L. and FRANZBLAU, S. G. Leprosy. Clin. Microbiol. Rev. 1(1988)330-348.
11. ISAKOV, N. and ALTMAN, A. Human T lymphocyte activation by tumor promoters: role of protein kinase C.J. Immunol. 138(1987)3100-3107.
12. ISLAS, R. A., MORALES, O. R., FAFUTIS M. M., GONZALEZ, M. A. and ORTIZ, O. L. Deficiency in the biosynthesis of interleukin 2 (IL-2) and functional presence of the IL-2 receptor in lepromatous leprosy. Int. J. Lepr. 55(1987)566-569.
13. KORETSKY, G. A., DANIELE, R. P., GREENE, W. C. and NOWELL, P. C. Evidence for an interleukin-independent pathway for human lymphocyte activation. Proc. Natl. Acad. Sci. U.S.A. 80(1983)3444-3447.
14. LI, W., WHALEY, C. D., MONDINO, A. and MUELLER, D. Blocked signal transduction to the ERK and JNK protein kinases in anergic CD4+ T cells. Science 271(1996)1272-1276.
15. MEAGER, A. Cytokines. Buckingham, U.K.: Open University Press, 1990.
16. MILLS, G. B., CHEUNG, R. K., GRINSTEIN, S. and GELEAND, E. W. Increase in cytosolic free calcium concentration is an intracellular messenger for the production of interleukin 2 but not for the expression of the interleukin 2 receptor. J. Immunol. 134(1985)1640-1643.
17. MILLS, G. B., MAY, C, HILL, M. and GELFAND, E. W. Role of protein kinase C in interleukin 1, anti-T3, mitogenic lectin-induced interleukin 2 secretion. J. Cell. Physiol. 141(1989)310-317.
18. MILLS, G. B., STEWART, D. J., MELLORS, A. and GELFAND, E. W. Interleukin 2 does not induce phosphatidylinositol hydrolysis in activated T cells. J. Immunol. 136(1986)3019-3024.
19. MOHAGHEGHPOUR, N., GELBER, R. H. and ENGLE-MAN, E. G. T cell defect in lepromatous leprosy is reversible in vitro in the absence of exogenous growth factors. J. Immunol. 138(1987)570-574.
20. Quantikine Human IL-2 Immunoassay. Minneapolis, Minnesota, lis, Minnesota, U.S.A.: R & D Systems, 1994.
21. RIDLEY S. and JOPLING, W. H. Classification of leprosy according to immunity: a five-group system. hit. J. Lepr. 34(1966)255-273.
22. SCHWARTZ, R. H. Anergia de las celulase T. Invest. Cien. 205(1993)24-31.
23. SZAMEL, M., REHERMANN, B., KREBS, B., KURRIE, R. and RESH, K. Activation signals in human lym-phocytes. J. Inummol. 143(1989)2806-2813.
24. TRUNEH, A., ALBERT, F., GOLSTEIN, P. and SCHMITT-VERHUIT, A. M. Early steps of lympho-cyte activation bypassed by synergy between calcium ionophores and phorbol ester. Nature 313(1985)318-320.
1. M.Sc. Laboratorio de Inmunobiologia, Departamento Biologia Celular y Molecular, División Ciencias Biológicas y Ambientales, Centro Universitario de Ciencias Biológicas y Agropecuarias, Universidad de Guadalajara, Guadalajara, Jalisco, México.
2. M.T., Centro de Investigación en Inmunología y Dermatologia, Universidad de Guadala¡ara/Instituto Dermatológica de Jalisco, Apartado Postal 5-149, CP. 45040 Col. Chapalita. Guadalajara, Jalisco, México.
3. M.D., Centro de Investigación en Inmunología y Dermatologia, Universidad de Guadala¡ara/Instituto Dermatológica de Jalisco, Apartado Postal 5-149, CP. 45040 Col. Chapalita. Guadalajara, Jalisco, México.
4. M.Sc, Laboratorio de Inmunobiologia, Departamento Biologia Celular y Molecular, División Ciencias Biológicas y Ambientales, Centro Universitario de Ciencias Biológicas y Agropecuarias, Universidad de Guadalajara, Guadalajara, Jalisco, México.
5. M.Sc., Centro de Investigación en Inmunología y Dermatologia, Universidad de Guadala¡ara/Instituto Dermatológica de Jalisco, Apartado Postal 5-149, CP. 45040 Col. Chapalita. Guadalajara, Jalisco, México.
Reprint requests to Mary Fafutis-Morris.
Received Cor publicution on 13 February 1995.
Accepted for publication in revised form on 20 November 1996.