- Volume 65 , Number 1
- Page: 1–11
Low serum HDL-cholesterol is associated with raised tumor necrosis factor-α during ENL reactions
ABSTRACT
The concentrations of serum lipids and tumor necrosis factor (TNF) were measured in leprosy patients across the spectrum of the disease and in erythema nodosum leprosum (ENL) patients at the onset of the reaction and after the reaction had clinically subsided. Lepromatous/borderline lepromatous (LL/BL) patients had significantly higher serum triglyceride and lower HDLcholesterol levels; there was no such change in the tuberculoid/borderline tuberculoid (TT/BT) patients. The household contacts (HC) of the LL/BL patients also had significantly lower serum HDL levels. ENL patients during the acute phase of the reaction had significantly lower total, LDL-, HDL-cholesterol levels compared to the stable LL/BL patients, and these changes were reversible to pre-ENL levels after the reaction had subsided. Serum TNF levels were significantly higher in household contacts and in LL/BL patients but were not statistically different in TT/BT patients. Serum TNF levels were also significantly higher during the acute phase of ENL, and declined after the clinical remission of the reaction to levels comparable with those of LL/BL patients. There was a significant negative correlation between serum TNF and HDL-cholesterol levels during and after ENL reaction. However, there was no such correlation between TNF and total or LDL-cholesterol leveis in ENL patients. Our results suggest that the changes in HDL-cholesterol mctabolism are a spécifie part of the host response to lepromatous leprosy and to the ENL reaction, and may be mediated by inereased TNF production.RÉSUMÉ
Les concentrations de lipides sériques et de facteur nécrosant des tumeurs (FNT) ont été mesurées chez des malades de la lèpre dans tout le spectre de la maldie et chez des patients présentant un érythème noueux lépreux (ENL) au début de la réaction et après que la réaction ait cliniquement disparu. Les patients lépromateux/borderline (LL/BL) avaient des taux de triglycérides sériques significativement plus élevés et des taux de HDL-cholestérol significativement plus bas. Il n'y avait pas de telles modifications chez les patients tiberculoides/borderlines (TT/BT). Les contacts domiciliaires des patients LL/BL avaient également des taux de HDL sérique significativement plus bas. Les patients avec un ENL en phase aiguë avaient des taux totaux, de LDL- et de HDL-cholesterol significativement plus faibles, comparés aux patients LL/BL en état stable; ces modifications étaient réversibles pour revenir aux niveaux d'avant la réaction une fois que celle-ci avait disparu. Les taux sériques de FNT étaient significativement plus élevés chez les contacts domiciliaires des patients LL/BL, mais n'étaient pas statistiquement différents chez les patients TT/BT. Les taux sériques de FNT étaient également significativement plus élevés durant la phase aiguë de l'ENL, et diminuaient, après la rémission clinique de la réaction, à des niveaux comparables à ceux des patients LL/BL. Il y avait une correlation négative significative entre les taux sériques de FNT et de HDL-eholestérol durant et après la réaction d'ENL. Cependant, il n'y avait pas une telle correlation entre les taux de FNT et le cholestérol total ou LDL chez les patients avec un ENL. Nos résultats suggèrent que les modifications dans le métabolisme du HDL-eholestérol sont une composante spécifique de la réponse de l'hôte à la lèpre lépromateuse et à la réaction ENL, et peut être médiée par une production accrue de FNT.RESUMEN
Se midieron las concentraciones de los lípidos y de factor de necrosis tumoral (TNF") en el suero de pacientes con lepra sin reacción y en pacientes con eritema nodoso leproso (ENL), al inicio de la reacción leprosa y después de que ésta hubo remitido. Los pacientes lepromatosos y los lepromatosos subpolares (LL/BL) tuvieron los niveles de triglicéridos más altos y los menores niveles de 1 IDL-colesterol; en los pacientes tuberculoides y tuberculoid.es subpolares (TT/BL) no hubieron tales cambios. Los contactos familiares de los pacientes LL/BL también tuvieron niveles de HDL significativamente disminuidos. Los pacientes con ENL tuvieron niveles significativamente más bajos de colesterol total, de LDL- y IIDL-colesterol, que los pacientes LL/BL estables, y estos cambios regresaron a los niveles encontrados antes de la reacción, después de que ésta hubo remitido. Los niveles de TNE fueron significativamente más altos en los contactos familiares que en los pacientes LL/BL pero no fueron estadísticamente diferentes en los pacientes TT/BT. Los niveles de TNF en suero también estuvieron significativamente elevados durante la fase aguda del ENL y decayeron después de la remisión clínica de la reacción, a niveles comparables a los encontrados en los pacientes LL/BL. Se encontró una correlación negativa significativa entre los niveles de TNF y los de HDL-colesterol durante y después de la reacción ENL. Sin embargo, no se observó tal correlación entre los niveles de TNF y de HDL-colesterol en los pacientes con ENL. Nuestros resultados sugieren que los cambios en el metabolismo del HDL-colesterol son una parte específica de la respuesta del huésped a la lepra lepromatosa y a la reacción ENL, y que estos cambios pueden estar mediados por la incrementada producción de TNF.Leprosy is a chronic infectious disease that is characterized by a broad spectrum of clinical forms depending upon the immune response of the host to Mycobacterium leprae (32). At one end of the spectrum there is lepromatous leprosy which is associated with impaired cell-mediated immunity (CMI) and an uncontrolled replication of the mycobacteria. At the other end of spectrum there is tuberculoid leprosy which is associated with pronounced CMI and low numbers of mycobacteria in skin macrophages (33).
Erythema nodosum leprosum (ENL) is an acute reactional complication commonly seen in patients with lepromatous disease and is associated with local and systemic inflammation (3I). The host response to infectious agents and inflammatory stimuli is usually accompanied by stimulation of acute phase protein synthesis and several alterations in lipid metabolism (2,5,9,11,21,39,45). It has been shown that the levels of C-reactive protein (CRP) and serum amyloid A (SAA) are markedly elevated in ENL patients as compared to nonreactional lepromatous patients and endemic controls (13,18,24,43) indicating that the synthesis of acute phase proteins is stimulated during ENL. We have also shown that lepromatous/borderline lepromatous (LL/BL) patients have higher serum triglyceride and lower HDL-cholesterol levels compared to endemic controls; whereas ENL patients have markedly lower total, HDL-, and LDL-cholesterol levels compared to LL/BL patients (28).
The stimulation of acute phase protein synthesis and the changes in lipid metabolism during infection and inflammation are now thought to be mediated by cytokines (26-37). Tumor necrosis factor (TNF), interleukin-1 (IL-1), and IL-6 induce hepatic acute phase protein synthesis (30,36). Similarly TNF, IL-1 and IL-6 increase hepatic lipid synthesis and decrease lipoprotein lipase activity, resulting in an increase in serum triglyceride levels (10,25). TNF also decreases HDL-cholesteroI levels in rodents as well as primates (l7), and has been proposed to be a mediator of changes in lipid and lipoprotein metabolism in experimental infection models (27). Serum TNF levels are markedly elevated during ENL, and it is thought to play a major role in the pathogenesis of ENL (4,34,42).
Our earlier studies compared the changes in serum lipid levels between patients with lepromatous disease and ENL (:s). Now we have extended these studies to include leprosy patients across the spectrum of the disease as well as recently exposed, healthy household contacts to assess acute phase serum markers associated with leprosy which may be used as predictors for reactions. We have also examined changes in serum lipoprotein levels in a new cohort of ENL patients at the onset of reaction and after the reaction had clinically subsided. Additionally, we have measured serum TNF concentrations in these patients and have examined the correlation between TNF and changes in serum lipoprotein levels during and after ENL reaction.
MATERIALS AND METHODS
Study subjects. The patient group for this study consisted of 27 untreated leprosy patients with no known history of any reactional episode. The untreated leprosy patients were further classified as lepromatous (LL/BL, N = 17, mean age 33 years, 15 males and 2 females) or tuberculoid (BT/ TT, N = 10, mean age 28 years, 3 males and 7 females) according to the standard criterion of Ridley and Jopling (38). The study group also included 12 histologically confirmed ENL patients. The mean age of the ENL patients was 35 years (range 17-60 years) with a male : female ratio of 3:1. All of the ENL patients had severe reactions and needed hospitalization. They were treated symptomatically with analgesics and nonsteroidal antiinflammatory drugs. Three of the ENL patients received steroid treatment; only one received thalidomide treatment. The characteristics of the ENL patients including age, sex, bacterial index, history of previous ENL episodes, duration of reaction, pre- and post-reaction treatment are summarized in Table 1.
The control group for this study included 14 household contacts of LL/BL patients with no apparent clinical signs of leprosy and 22 healthy endemic controls who had no known contact with leprosy patients and were employed at The Aga Khan University Medical College.
Serum TNF concentrations. Venous blood samples were collected from all patients in glass tubes without any anticoagulants. In ENL patients the blood samples were collected before initiation of treatment for the reaction and after clinical remission of the reaction. In lepromatous and tuberculoid patients blood samples were taken at the time of admission. The blood was allowed to clot at room temperature, and the samples were centrifuged to remove red blood cells. Serum was separated and stored in aliquots at -70°C. Before the assay for TNF estimation, the samples were thawed at room temperature. TNF concentrations were measured according to the manufacturer's instructions using a commercially available human TNF- α ELISA (Quantikine HS TNF- α , R&D Systems, Minneapolis, Minnesota, U.S.A.). This TNF ELISA has a sensitivity of 0.5 pg/ml. A standard curve was obtained by plotting the readings of the standard TNF solution supplied with the kit and the individual readings were obtained by comparing readings with the standard curve.
Serum lipid concentrations. Serum triglyceride and total cholesterol levels were measured by standard enzymatic assays as described earlier (27). Serum HDLcholesterol was measured cnzymatically after precipitation of chylomicrons, VLDL and LDL with phosphotungistic acid and magnesium chloride (1). Serum LDL levels were determined by the method of Friedwald, etal. (14).
Statistical analysis. The data are presented as mean ± standard error of the mean (S.E.M.). The level of significance between the control subjects and the different patient groups was determined by Student's unpaired / test. The paired t test was used to compare the differences between ENL patients during and after the reaction; p values < 0.05 were considered statistically significant. Linear regression analysis was performed to determine the correlation between serum TNF and lipoprotein concentrations.
RESULTS
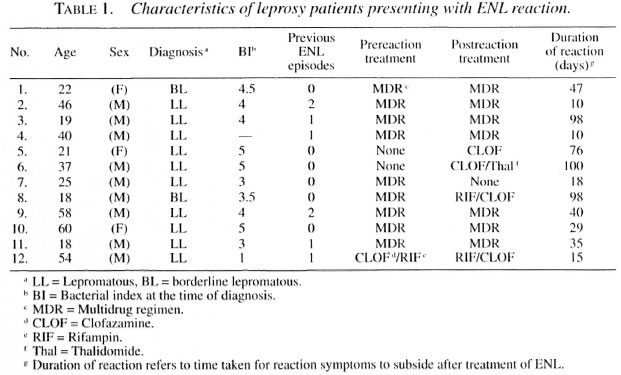
Serum lipid concentration in lepromatous, tuberculoid and ENL patients. The data presented in Figure 1 compare serum lipid levels in endemic controls (EC), household contacts (HC) of LL/BL patients, LL/BL and BT/TT patients. The HC group had significantly lower total (p < 0.005) and HDL-cholesterol levels (p < 0.0001) compared to the EC group; there were no significant differences in serum triglyceride and LDL levels. Serum triglyceride levels were increased by 35% over the EC group in LL/BL patients (p < 0.04). There were no significant differences in serum total or LDL-cholesterol concentrations between the EC and LL/BL groups; however, HDL levels were decreased by 32% in LL/BL patients (p < 0.0001). These results confirm our earlier observation of changes in triglyceride and HDL levels associated with lepromatous disease (28) in a new cohort of LL/BL patients. Unlike the HC and LL/BL groups, there were no statistically significant differences in serum triglycerides, total, LDL-, or HDL-cholesterol levels in tuberculoid patients as compared to controls, indicating that the changes in lipid metabolites are specific and are primarily observed in contacts of and patients with lepromatous disease.
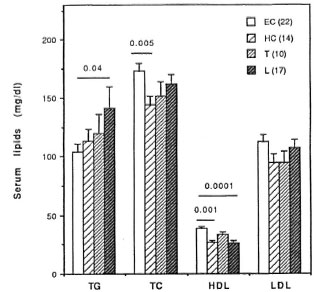
Fig.1. Serum triglyceride (TG), total cholesterol(TC), I IDL and LDL levels in endemic controls (EC), household contacts of LL/BL patients (HC), untreated tuberculoid (T) and lepromatous (L) patients. Number of subjects in each group is given in parentheses. Serum lipid levels were measured using standard enzymatic assays as described under Materials and Methods.
We next compared the changes in the serum lipid concentrations in LL/BL patients with those presenting with acute ENL and again after the clinical remission of the ENL reaction. The data presented in Figure 2 show that as compared to LL/BL patients, significant decreases are observed in total (p < 0.002), LDL- (p < 0.03) and HDL-cholesterol (p < 0.0001) concentrations in patients presenting with acute ENL reaction. The changes produced in the serum lipid levels during the acute phase of ENL are transient since serum total, LDL-, and HDL-cholesterol levels in ENL patients after the reaction had subsided clinically are comparable to those in LL/BL patients and are significantly higher than the levels during the course of the acute ENL reaction (Fig. 2).
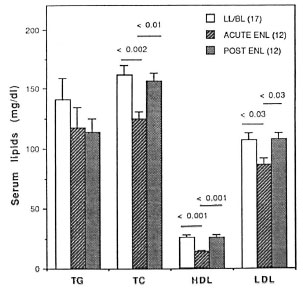
Fig. 2. Comparison of serum triglyceride (TG), total cholesterol (TC), HDL and LDL levels in LL/BL patients with ENL patients during the acute phase of reaction (acute ENL) and after the clinical remission of ENL (post-ENL). Number of subjects in each group isgiven in parentheses. Serum lipid levels were measured using standard enzymatic assays as described under Materials and Methods.
The data presented in Figure 3 depict the HDL-cholesterol values in individual subjects in the control groups, Iepromatous and tuberculoid patients and during and after ENL reactions. These data clearly demonstrate that despite a wide variation in HDL levels in the endemic controls, the serum HDL levels were markedly lower in contacts of and patients with Iepromatous disease (p < 0.0001 ) and were even further decreased during the course of ENL reaction (p< 0.0001).
Fig. 3. Serum HDL-cholesterol levels in individual control subjects, leprosy patients and during and after ENL reaction. Numbers in parentheses represent number of control subjects or patients in each group; horizontal lines show medians for each group.
Serum TNF levels in Iepromatous, tuberculoid and ENL patients. The data presented in Figure 4 show that serum TNF levels were increased significantly (p < 0.001) in the HC group when compared to the EC group. The serum TNF levels were 2.4-fold higher in the BT/TT patients as compared to the EC group; however, this increase was not statistically significant. There was a 3.8-fold increase (p < 0.004) in serum TNF levels over the EC group in the LL/BL patients. Serum TNF levels were 5.9-fold higher (p < 0.0001) in ENL patients as compared to the EC group during the reaction (before the initiation of treatment) and 2.8-fold higher (p < 0.004) after the treatment (post-ENL) when the reaction had clinically subsided.
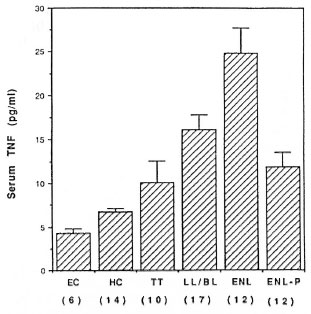
Fig. 4. Serum tumor necrosis factor (TNF) levels across the spectrum of leprosy and during and after ENL reaction. Numbers in parentheses represent number of patients or controls in each group. Serum TNF levels were measured by an ELISA method as described under Materials and Methods.
A comparison of serum TNF levels in ENL and stable LL/BL patients revealed that the TNF levels were 54% higher (p < 0.009) during the acute phase of the ENL reaction and were not significantly different after the reaction had clinically subsided. Table 2 summarizes the level of significance for serum TNF concentrations among different groups across the disease spectrum and during and after ENL reaction.
Correlation between serum TNF and lipid concentrations. Since TNF has been shown to mediate the changes in lipoprotein metabolism in rodents (27), we examined the correlation between serum TNF and changes in serum total, LDL-, and HDLcholesterol levels during acute ENL and after the clinical remission of the ENL reaction by linear regression analysis (Fig. 5).
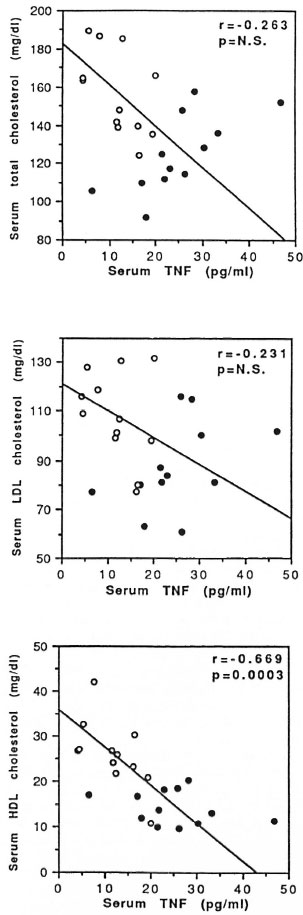
Fig. 5. Correlation between serum tumor necrosis factor and total (top panel). LDL,- (middle panel) and HDL-cholesterol (bottom panel) concentrations inENL, patients at the time of admission ( = acute ENL, N = 12) and after the reaction subsided clinically ( = post-ENL, N = 12). Linear regression analysis was used to determine the r and p values for correlation and level of significance.
= post-ENL, N = 12). Linear regression analysis was used to determine the r and p values for correlation and level of significance.
The data presented indicate that there is a significant negative correlation (r = -0.669, p = 0.0003) between HDL-cholesterol and TNF levels during and after ENL reaction. A majority of the patients during ENL reaction had higher TNF and lower HDL levels, and this trend was reversed after the reaction had clinically subsided. On the other hand, although there was a trend toward higher TNF and lower total and LDL-cholesterol levels during and after ENL reaction, the correlation did not reach statistical significance.
DISCUSSION
The host response to a variety of bacterial, viral and parasitic infections is usually accompanied by several disturbances in lipid and lipoprotein metabolism (2,5,9,11,39). We have recently shown that patients with lepromatous disease have higher serum triglyceride and lower HDL-cholesterol levels (28). The present study confirms and extends these findings across the spectrum of leprosy. Our results demonstrate that while LL/BL patients had significantly higher serum triglyceride and lower HDLcholesterol levels similar changes were not observed in BT/TT patients, indicating that these changes in lipid metabolism produced in patients with lepromatous disease are not a nonspecific host response in leprosy but are unique to LL/BL patients.
It is well established that the household contacts of leprosy patients have a high risk of developing the disease as compared to endemic controls (46). The relative risk is three to six times higher for contacts of multibacillary (MB) patients and two to four times higher for contacts of paucibacillary (PB) patients than the general population (46). Identification of individuals at risk for developing the disease is crucial for the effective control of leprosy and its prevention. At present there are no simple biochemical or immunological markers which can be used to identify the high-risk population. The data presented here demonstrate for the first time that the most consistent and probably the earliest response in healthy household contacts of LL/BL patients is a decrease in serum HDL-cholesterol levels. Among the household contacts of LL/BL patients, 10/14 had a serum HDL concentration below 30 mg/dl. This was similar to the LL/BL group in which 12/17 had serum HDL levels below 30 mg/dl. The magnitude of the decrease in HDL concentrations in household contacts was also very similar to that seen in LL/BL patients, indicating the specificity of this host response in multibacillary leprosy. Whether a decrease in serum HDL concentrations, which is a very specific metabolic host response in household contacts of LL/BL patients, can be used as a sensitive biochemical marker for predicting the high-risk population for the development of leprosy is debatable and certainly needs long-term longitudinal studies in a large group of healthy household contacts and patients.
We have previously also shown that ENL patients have markedly lower serum total, LDL-, and HDL-cholesterol levels as compared to patients with stable lepromatous disease (28). Since ENL can occur before the initiation of therapy, during the course of treatment, or subsequent to completion of therapy (31) (which may also influence serum lipoprotein levels), here we examined changes in serum lipoprotein levels in a new cohort of patients presenting with ENL before the initiation of treatment and after the reaction had clinically subsided. The results of the present study confirm our earlier observations of markedly decreased total, LDL-, and HDL-cholesterol concentrations during ENL as compared to untreated lepromatous patients, and further demonstrate that these changes in lipoprotein levels are reversible since there was no difference between lipoprotein levels in ENL patients after the clinical remission of reaction and stable LL/BL patients.
Several previous studies have measured serum TNF levels across the leprosy spectrum (34,35,42,44) and TNF production by peripheral blood mononuclear cells (PBMC) from leprosy patients in response to Mycobacterium leprae (4,41); however, the data have been quite inconsistent. While some of these studies have reported higher serum TNF levels in tuberculoid leprosy (44), others have shown high TNF levels in patients with lepromatous disease (34,35). Sarno, et al. (42) reported widely variable serum TNF levels across the leprosy spectrum and attributed discrepancies in serum TNF levels in previous studies to the differences in the methods used for estimation of TNF since radioimmunoassay (4,34,42), ELISA (4,35) or cytotoxicity assays (4,41,44) were used in different studies. The differences in TNF levels measured with bioassays and immunoassays could be due to the presence of TNF inhibitors, TNF soluble receptors or biologically inactive TNF (6,12). However, Barnes, el al. (4) have shown that there is excellent agreement among the results obtained by these three assays for TNF estimation and the trends are generally consistent across different patient groups for each assay.
In this study we have used a highly sensitive human TNF-α ELISA with a detection limit of 0.5 pg/ml. Our results demonstrate that serum TNF levels are significantly higher in household contacts of LL/BL patients and in patients with lepromatous disease. On the other hand, while there is a trend toward higher serum TNF levels in tuberculoid patients, it did not reach the level of statistical significance probably due to higher variability among our patients. Our results are in agreement with those of Parida, et al. (34) and Pisa, et al. (35), who reported significantly high serum TNF levels in LL/BL patients. On the other hand, our results are in marked contrast with those of Silva and Foss (44) who showed that 75% of tuberculoid patients had high serum TNF levels whereas all lepromatous patients had TNF levels that were comparable to those of endemic controls. The median duration of treatment in their lepromatous patients was above 5 years, and this could be the reason for low TNF levels in these patients. Sampaio, et al. (40) have shown that serum TNF levels are decreased in LL/BL patients undergoing multidrug therapy due to a reduction in the bacterial burden. All of our lepromatous as well as our tuberculoid patients were newly diagnosed and previously untreated, and this may also account for the apparent discrepancies in serum TNF levels between different studies.
The data on serum TNF levels as well as TNF production by PBMC in ENL patients are more consistent. All of the studies have shown raised serum TNF levels (34,42) or a high spontaneous or stimulated TNF production by PBMC from patients with ENL (4,41). Our results, showing significantly higher serum TNF levels during the acute phase of ENL reaction, are in agreement with these studies and further demonstrate that treatment of ENL can lower serum TNF levels. In our study, serum TNF levels in ENL patients after the reaction had subsided clinically were comparable to those in stable LL/BL patients. It has been shown that treatment with thalidomide can reduce serum TNF levels in ENL patients (40) perhaps by enhancing TNF mRNA degradation (29). Our results indicate that serum TNF levels usually decrease after the clinical remission of reaction regardless of the mode of treatment.
Several cytokines, including TNF, IL-1, IL-6, interferon-α and interferon-γ, have been shown to produce a variety of alterations in lipid metabolism (26). However, there is a paucity of data on cytokine mediators of metabolic disturbances in human diseases. Previous studies have shown that TNF is the mediator of endotoxin-induced changes in lipoprotein metabolism in rodents (27); hence here we looked at the correlation between serum TNF and lipoprotein levels in ENL patients during the acute phase and after the reaction had clinically subsided. Our results demonstrate that there is a significant negative correlation between serum TNF and HDL-cholesterol levels, suggesting that an increase in TNF production during the course of ENL reaction may be responsible for the decrease in serum HDL levels. It is likely that cytokine mediators of various metabolic disturbances could be different in different diseases. Grunfeld, et al. (15,16) have shown that there is a significant positive correlation between serum interferon-α and hypertriglyceridemia in patients with AIDS.
Although we did not examine the mechanisms that could decrease serum HDL levels in patients with lepromatous disease or during ENL reaction, several in vitro and in vivo studies have been conducted to determine the mechanisms by which TNF could lower serum HDL levels. TNF decreases the production of apo-Al, the major apoprotein that is associated with HDL, in Syrian hamsters (17) and HepG2 cells (8), and a decrease in apo-A 1 production may lower serum HDL levels. TNF could also lower HDL levels by enhancing the degradation of HDL by macrophages (19) or by decreasing lecithin cholesterol acyltransferase (LCAT) activity (8,23). Finally, TNF increases the hepatic expression as well as the serum levels of SAA (7,30') which is an acute phase protein and is bound to HDL in the circulation (3,22) An increase in SAA has been shown to redirect the metabolism of HDL from hepatocytes toward macrophages at the site of inflammation (20), resulting in a decrease in serum HDL levels during the acute phase response. We have earlier shown a significant negative correlation between SAA and HDL levels in LL/BL and ENL patients (28) and have proposed that an increase in SAA may lower serum HDL levels in ENL by redirecting its metabolism. Which of these potential TNF-mediated mechanisms is/are involved in producing the changes in HDL-cholesterol metabolism in lepromatous leprosy and ENL reactions is not clear at this stage and needs further mechanistic studies.
Acknowledgment. This investigation was supported by research funds from The Rockefeller Foundation (RH), UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (RH) and The Aga Khan University Seed Money Grant (RAM). We would like to thank Drs. Z. Uquaili and Q. Ahsan for providing the clinical material. We thank Ms. M. Dojki for technical assistance and Mr. II. Akram for help with statistical analysis and graphics. Secretarial assistance of Ms. R. Paul in manuscript typing is gratefully acknowledged.
REFERENCES
1. ALBERS, J. J., WARNICK, G. R. and WIEBE, D. Multi-laboratory comparison of three heparin-Mn2+ precipitation procedures for estimating cholesterol in high density lipoprotein. Clin. Chem. 24(1978)853-856.
2. ALVAREZ, C. and RAMOS, A. Lipids, lipoproteins and apoproteins in serum during infection. Clin. Chem. 32(1986)142-145.
3. BANKA, C. L., YUAN, T., DE BEER, M. C, KINDY, M., CURTISS, L. K. and De BEER, F. C. Serum amyloid A (SAA): influence on HDL-mediated cellular cholesterol efflux. J. Lipid Res. 36(1995)1058-1065.
4. BARNES, P. F, CHATTERJEE , D., BRENNAN, P. J.. REA, T. H. and MODLIN, R. L. Tumor necrosis factor production in patients with leprosy. Infect. Immun. 60(1992)1441-1446.
5. CABANA, V. G., SIEGEL, J. N. and SABESIN, S. M. Effects of the acute phase response on the concentration and density distribution of plasma lipids and apolipoproteins. J. Lipid Res. 30(1989)39-49.
6. CAVAILLON, J., MUNOZ, C, FITTING, C, MISSET , B. and CARLET, J. Circulating cytokines: the tip of the iceberg. Circ. Shock 38(1992)145-152.
7. DOWTON, S. B., PETERS, C. N. and JESTUS , J. J. Regulation of serum amyloid A gene expression in Syrian hamsters by cytokines. Inflammation 15(1991)391-397.
8. ETTINGER , W. H., VARMA , V. K., SORCI -THOMAS, M., PARKS , J. S., SIGMON, R. C. SMITH, T. K. and VERDERY, R. B. Cytokines decrease apolipoprotein accumulation in medium from HepG2 cells. Arterioscler. Thromb. 14(1994)8-13.
9. FEINGOLD , K. R., HARDARDOTTIR, I.. MEMON, R. A., KRUL, E. T. J., MOSER, A. H., TAYLOR , J. M. and GRUNFELD, C. Effect of endotoxin on cholesterol biosynthesis and distribution in serum lipoproteins in Syrian hamsters. J. Lipid Res. 34(1993)2147-2158.
10. FEINGOLD , K. R., SOUED, M., SERIO, M. K., MOSER, A. H., DINARELLO, C. A. and GRUNFELD, C. Multiple cytokines stimulate hepatic lipid synthesis in vivo. Endocrinology 125(1989)267-274.
11. FEINGOLD , K. R., STAPRANS, I., MEMON, R. A., MOSER, A. H., SHIGENAGA , J. K., DOERRLER , W., DlNARELLO, C. A. and GRUNFELD, C. Endotoxin rapidly induces changes in lipid metabolism that produce hypertriglyceridemia: low doses stimulate hepatic triglyceride production while high doses inhibit clearance. J. Lipid Res. 33(1992)1765-1776.
12. FOMSGAARD, A., WOLSAAE, M. and BENDTZEN, K. Detection of tumor necrosis factor from lipopolysaccharide stimulated human mononuclear cells by enzyme linked immunosorbent assay and cytotoxicity bioassay. Scand. J. Immunol. 27(1988)143-147.
13. FOSS, N. T, OLIVEIRA, E. B. and SILVA, C. L. Correlation between TNF production, increase of plasma C-reactive protein levels and suppression of T-lymphocyte response to concanavalin-A during erythema nodosum leprosum. Int. J. Lepr. 61(1993)218-226.
14. FRIEDEWALD , W. T, LEVY, R. 1. and FREDRICKSON, D. S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma without the use of preparative ultracentrifuge. Clin. Chem. 18(1972)499-502.
15. GRUNFELD, C, KOTLER, D. P., SHIGENAGA , J. K., DOERRLER , W., TIERNEY, A., WANG , J., PIERSON, R. N. and FEINGOLD , K. R. Circulating interferon-α levels and hypertriglyceridemia in the acquired immunodeficiency syndrome. Am. J. Med. 90(1991)154-162.
16. GRUNFELD, C, PANG, M.. DOERRLER, W., SHIGENAGA , J. K., JENSIN , P. and FEINGOLD, K. R. Lipids, lipoproteins, triglyceride clearance and cytokines in human immunodeficiency virus infection and the acquired immunodeficiency syndrome. J. Clin. Endocrinol. Metab. 74(1992)1045-1052.
17. HARDARDOTTIR, I., MOSER, A. H., MEMON, R. A. GRUNFELD, C. and FEINGOLD, K. R. Effects of TNF, IL-1, and the combination of both cytokines on cholesterol metabolism in Syrian hamsters. Lymphokine Cytokine Res. 13(1994)161-166.
18. HUSSAIN, R.,LUCAS, S. B., KIFAYET, A., JAMAL., S., RAYNES, J., UQAILI, Z., DOCKRELL, H. M., CHIANG, T. J. and McADAM, K. P. W. J. Clinical and histological discrepancies in diagnosis of ENL reactions classified by assessment of acute phase proteins SAA and CRP. Int. J. Lepr. 63(1995)222-230.
19. KEIDAR, S., GILHAR, A., KAPLAN, M., BROOK, J. B. and AVIRAM, M. Enhanced degradation of high density lipoprotein by peritoneal macrophages from nude mice is attenuated by interleukin-l. Artery 20(1993)268-279.
20. KISILEVSKY, R. and SUBRAHAMANYAN, L. Serum amyloid A changes high density lipoprotein's cellular affinity; a clue to serum amyloid A's principal function. Lab. Invest. 66(1992)778-785.
21. KUSHNER, I. The acute phase response: from Hippocrates to cytokine biology. Eur. Cytokine Net. 2(1991)75-80.
22. LIANG, J. and SIPE:, J. D. Recombinant human serum amyloid A (apo SAAp) binds cholesterol and modulates cholesterol flux. J. Lipid Res. 36(1995)37-46.
23. LY, H., FRANCONE, O. L., FIELDING, C. J., SHIGENAGA, J. K., MOSER, A. H., GRUNFELD, C. and FEINGOLD, K. R. Endotoxin and TNE lead to reduced plasma LCAT activity and decreased hepatic LCAT mRNA levels in Syrian hamsters. J. Lipid Res. 36(1996)1254-1263.
24. MCADAM, K. P. W. J., ANDERS, R. F., SMITH, S. R., RUSSEL, D. A. and PRICE, M. A. Association of amyloidosis with erythema nodusum leprosum reactions and recurrent neutrophil leucocytosis in leprosy. Lancet 2(1975)572-576.
25. MEMON, R. A., FEINGOLD, K. R., ADI, S. and GRUNFELD, C. Which cytokines mediate the metabolic effects of endotoxin? In: Tumor Necrosis Factor. Vol. 4. Fiers, W. and Burman, W., eds. Basel: Karger, 1993, pp. 107-112.
26. MEMON, R. A., FEINGOLD, K. R. and GRUNFELD, C. Cytokines, lipid metabolism and cachexia. In: Human Cytokines: Their Role in Disease and Therapy. Aggarwal, B. B. and Puri, R. K., eds. Cambridge: Blackwell Scientific, 1995, pp. 239-251.
27. MEMON, R. A., GRUNFELD, C, MOSER, A. H. and FEINGOLD, K. R. Tumor necrosis factor mediates the effects of endotoxin on cholesterol and triglyceride metabolism in mice. Endocrinology 132(1993)2246-2252.
28. MEMON, R. A., HUSSAIN, R., RAYNES, J. G., LATEEF, A. and CHIANG, T. G. Alterations in serum lipids in lepromatous leprosy patients with and without ENL reactions and their relationship to acute phase proteins. Int. J. Lepr. 64(1996)115-122.
29. MOREIRA, A. L., SAMPAIO, E. P., ZMUIDZINAS, A., FRINDT, P., SMITH, K. A. and KAPLAN, G. Thalidomide exerts its inhibitory action on tumor necrosis factor-α by enhancing mRN A degradation. J. Exp. Med. 177(1993)16715-1680.
30. MORTENSEN, R. F., SHAPIRO, J., LIN, B. F, DOUCHES, S. and NETA, R. Interaction of recombinant IL-I and recombinant tumor necrosis factor in the induction of mouse acute phase proteins. J. Immunol. 140(1988)2260-2266.
31. NAAFS, B. Leprosy reactions; new knowledge. Trop. Geogr. Med. 46(1994)80-84.
32. OTTENHOFF, T. H. M. Immunology of leprosy; new developments. Trop. Geogr. Med. 46(1994)72-80.
33. OTTENHOFF, T. H. M. Immunology of leprosy; lessons from and for leprosy. Int. .1. Lepr. 62(1994)108-121.
34. PARIDA, S. K., GRAU, G. E., ZAHEER, S. A. and MUKHERJEE, R. Serum tumor necrosis factor and interleukin-l in leprosy and during lepra reactions. Clin. Immunol. Immunopathol. 63(1992)23-27.
35. PISA, P., GENNENE, M., SODER, O., OTTENHOFF, T., HANSSON, M. and KlESSLING, R. Serum tumor necrosis factor levels and disease dissemination in leprosy and leishmaniasis. J. Infect. Dis. 161(1990)988-991.
36. RAYNES, J. G., EAGLING, S. and MCADAM, K. P. W. J. Acute phase protein synthesis in human hepatoma cells: differential regulation of serum amyloid A (SAA) and haptoglobin by interleukin-l and interleukin-6. Clin. Exp. Immunol. 83(1991)488-491.
37. RICHARDS, C. D. and GAULDIE, J. Role of cytokines in acute phase response. In: Human Cytokines: Their Role in Disease and Therapy. Aggarwal, B. B. and Puri, R. K., eds. Cambridge: Blackwell Scientific, 1995, pp. 253-269.
38. RIDLEY, D. S. and JOPLING, W. H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966)255-273.
39. SAMMALKORPI, K., VALTONEN, V., KERTTULA, Y., NIKKIL.A, E. and TASKINEN, M. R. Changes in serum lipoprotein pattern induced by acute infections. Metabolism 37(1988)859-865.
40. SAMPAIO, E. P., KAPLAN, G., MIRANDA, A., NERY, J. A. C, MIGUEL, C. P., VIANA, S. M. and SARNO, E. N. The influence of thalidomide on the clinical and immunologic manifestation of erythema nodosum leprosum. J. Infect. Dis. 168(1993)408-414.
41. SANTOS, D. O., SUFFYS, P. N., BONIFACIO, K., MARQUES, M. A. and SARNO, E. N. In vitro tumor necrosis factor production by mononuclear cells from lepromatous leprosy patients and from patients with erythema nodosum leprosum. Clin. Immunol. Immunopathol. 67(1993)199-203.
42. SARNO, E. N., GRAU, G. E, VIEIRA, L. M. M. and NERY , J. A. Serum levels of tumour necrosis factor-alpha and inlerletikin-1β J during leprosy reactional stales. Clin. Exp. Immunol. 84(1991)103-108.
43. SEHGAL., V. N., BATTACHARYA, S. N., SHAH, Y.,SHARMA, V. K. and GUPTA, C. K. Reactions in leprosy: acute phase reactant response during and after remission. Int. J. Dermatol. 31(1992)632-634.
44. SILVA, C. L,. and Foss, N. T. Tumor necrosis factor in leprosy patients. J. Infect. Dis. 159(1989)787-790.
45. SIPE, J. D. The acute-phase response. In: Inununophysiology: The Role of Cells and Cytokines Immunity and inflammation. Oppenheim J. J. and Shevach, E. M., eds. New York: Oxford University Press, 1990, pp. 259-273.
46. SUNDAR RAO, P S S , JESUDASAN, K., MANI, K.and CHRISTIAN, M. Impact of MDT on incidencerates of leprosy among household contacts; Part 1.baseline data. Int. J. Lepr. 57(1989)647-651.
1. M.B.B.S.. Ph.D.
2. M.Sc. Department of Physiology and Pharmacology, The Aga Khan University, Karachi, Pakistan.
3. M.Sc.
4. M. Phil.
5. Ph.D., M.R.C.(Path.), Department of Microbiology, The Aga Khan University, P. O. Box 3500, Karachi 74800, Pakistan.
6. M.B.B.S., M.Sc, The Marie Adelaide Leprosy Center, Karachi, Pakistan.
Reprint requests to Prof. Rabia llussain at above address or FAX 92-21-493-4294.
Received for publication on 24 April 1996; accepted for publication in revised form on 7 August 1996.