- Volume 65 , Number 1
- Page: 11–9
Regional lymphadenitis following antileprosy vaccine BCG with killed Mycobacterium leprae
ABSTRACT
Phase-II and extended Phase-II studies were conducted in three different sets of the population in Thiruthani taluk, Chengalpattu District, South India, involving BCG and killed Mycobacterium leprae (KML) combination vaccines to ascertain the acceptability of the vaccines. In the Phase-II study, 997 healthy volunteers were vaccinated on individual randomization with one of the vaccine arms: BCG 0.1 mg + 6 x 108 KML, BCG 0.1 mg + 5 x 107 KML, BCG 0.1 mg + 5 x 106 KML, BCG 0.1 mg or normal saline. Blood samples were taken and the serum was tested for antibody levels against phenolic glycolipid-I (PGL-I) and the 35-kDa protein of M. leprae. In this study, we observed regional suppurative adenitis in 6% (6 out of 100), 3% (3 out of 100), and 3% (3 out of 100) of the vaccinees in the BCG 0.1 mg + 6 x 108 KML, BCG 0.1 mg + 5 x 107 KML, and BCG 0.1 mg + 5 x 107 KML vaccine arms, respectively, in the 13-70-year age group. Earlier BCG scar status, skin-test reactions to lepromin-A, Rees' MLSA, and serum antibody levels against PGL-I and the 35-kDa protein did not help to identify the group at risk of developing suppurative adenitis. Suppurative adenitis appears to have a direct relationship between the age of the subject and the dose of the vaccine. In order to overcome the problem of regional suppurative adenitis and to know the mechanism involved, an extended Phase-II study was conducted in similar groups of the population by reducing the BCG and KML doses, i.e., with BCG 0.05 mg + 6 x 108 KML, BCG 0.05 mg + 5 x 107 KML, and BCG 0.01 mg + 5 x 107 KML. Biopsy specimens were collected f rom lymph nodes of the suppurative adenitis cases and were subjected for culture and histopathological examination. The observations showed that regional suppurative adenitis could be reduced to 1% in the BCG 0.05 + 6 x 108 KML group, 0.5% in the BCG 0.05 + 5 x 107 KML group, and 0.5% in the BCG 0.01<+ 5 x 107 KML group. This phenomenon of suppurative adenitis appears to be related to the total dose of mycobacterial antigens. Suppurative adenitis was seen by weeks 18 and 20 post-vaccination in the latter two lower doses; whereas it was seen by week 8 in the higher dose of the combination vaccines. No case of suppurative adenitis was observed in the BCG 0.1 mg group. Culture and histopathology ruled out the possibilities of progressive BCG infection and superadded infection. Considering the above results, BCG 0.05 mg + 6 x 108 KML was accepted for a large-scale vaccine trial in South India.RÉSUMÉ
On a réalisé des études de phase II et des études ex tensives de phase II avee des vaccins combinés BCG et Mycobacterium leprae tué (MCI) dans trois différents groupes de population au Thiruthani Taluk, dans le Distict de Chengalpattu dans le Sud de l'Inde, afin d'évaluer l'acceptabilité des vaccins. Dans l'étude de phase II, 997 volontaires en bonne santé ont été vaccinés en ayant reçu de manière aléatoire: BCG 0.1 mg + 6 x 108 MLT, BCG 0.1 mg + 5 x 107 MLT, BCG 0.1 mg + 5 x 106 MLT, BCG 0.1 mg ou du serum salin normal. On a pris des échantillons de sang et on a analysé le serum pour déterminer les taux d'anticorps contre le glycolipide phénolique I (PGL-I) et la protéine de 35 -kDa de M. leprae. Dans cette étude, nous avons observé une adénite suppurative régionale chez respectivement 6% (6 sur 100). 3% (3 sur 100) et 3% (3 sur 100) des personnes du groupe d'âge 13-70 ans vaccinées avec le BCG 0.1 mg + 6 x 108 MLT, BCG 0.1 mg + 5x I07 MLT, BCG 0.1 mg + 5 x 106MLT. La présence d'une cicatrice de BCG, les réactions au lest cutané à la lepromine A, au MLSA de Kees et les taux d'anticorps sériques vis-à-vis du PGL-I et de la protéine de 35 kDa n'ont pas aidé à identifier le groupe à risque de développer une adénite suppurative. Le développement d'une adénite suppurative apparaît être en relation directe avec l'âge du sujet cl la dose vaccinale. Afin de surmonter le problème d'adénite suppurative régionale et de connaître le mécanisme impliqué, nous avons conduit une étude extensive de phase II dans des groupes similaires de populations en réduisant les doses de BCG et de MLT, c'est à dire avec du BCG 0.05 mg + 6 x 108 MLT, BCG 0.05 mg + 5 x 107 MLT, BCG 0.01 mg + 5 x 107MLT. On a prélevé, pour culture et examen histopathologique, des biopsies des ganglions lymphatiques des cas présentant une adénite suppurative. Les observations ont montré que la fréquence de l'adénite suppurative régionale pouvait être réduite à 1% dans le groupe BCG 0.05 mg + 6 x 108 MLT, à 0.5% dans le groupe BCG 0.05 mg + 5 x 107 MLT, et à 0.5% dans le groupe BCG 0.01 mg + 5 x I07 MLT. Ce phénomène d'adénite suppurative apparaît associé à la dose totale d'antigène mycobactériens. L'adénite suppurative a été observée aux dix-huitième et vingtième semaines dans les deux derniers groupes aux doses plus faibles, alors qu'il a été observé à la huitième semaine avec la dose plus élevée de combinaison vaccinale. Aucun cas d'adénite suppurative n'a été observé dans le groupe ayant reçu le BCG 0.1 mg. La culture et I'histopathologic ont exclu la possibililé d'infection progressive par le BCG et d'inleclian surajoutée. Considérant les résultats cidessus, le BCG 0.05 mg + 6 x 10* MLT a été accepté pour un essai de vaccination à grande échelle dans le Sud de l'Inde.RESUMEN
Para establecer la aceptabilidad del BCG y de Mycobacterium leprae muerto por calor (MLMC) como vacunas contra la lepra, se realizaron estudios de tase II y de fase II ampliada en tres grupos de la población de Thiruthani taluk, Distrito de Chengalpattu, al sur de la India. En el estudio de fase II, se vacunaron 997 voluntarios sanos con uno de los siguientes esquemas de vacunación: 0.1 mg de BCG + 6 x 108 MLMC, 0.1 mg BCG + 5 x 107 MLMC, 0.1 mg de BCG + 5 x 106 MLMC, 0.1 mg de BCG, o solución salina fisiológica. Se tomaron muestras de sangre y el suero se utilizó para buscar anticuerpos contra el glicolípido fenólico-I (PGL-1) y el antigeno de 35 kDa de M. leprae. En el grupo de 13 a 70 años de edad, observamos adenitis supurativa regional en 6%, 3%, y 3% de los vacunados con BCG + 6 x 108 MLMC, BCG + 5 x I07 MLMC, y BCG + 5 x 106 MLMC, respectivamente. La presencia de cicatriz por BCG, las reacciones en piel a la lepromina A, el MLSA de Rees, y los niveles de anticuerpos anti-PGL-I y anti-35 kD, no ayudaron a identificar el grupo en riesgo de desarrollar adenitis supurativa. La adenitis supurativa parece tener una relación directa entre la edad del sujeto y la dosis de vacuna. Para resolver el problema de la adenitis supurativa regional y para conocer los mecanismos involucrados, se realizó un estudio de fase II ampliada en grupos similares de la población, utilizando dosis reducidas de vacunas BCG y MLMC (0.05 mg de BCG + 6 x 108 MLMC, 0.05 mg de BCG + 5 x 107 MLMC, y 0.01 mg de BCG + 5 x 107 MLMC). Se tomaron biopsias de ganglios linfáticos de los casos con adenitis supurativa y se usaron para cultivo y para examen histopatológico. Las observaciones mostraron que la adenitis supurativa regional pudo reducirse al 1 % en el grupo de 0.05 mg de BCG + 6 x 108 MLMC, al 0.5% en el grupo de 0.05 mg de BCG + 5 x 107 MLMC, y al 0.5% en el grupo de 0.01 mg de BCG + 5 x 107 MLMC. Este fenómeno de adenitis supurativa parece estar relacionado con la dosis total de los antígenos micobacterianos. La adenitis supurativa se observó entre las semanas 18 y 20 post-vacunación con las 2 dosis más bajas, mientras que ocurrió hacia la semana 8 con las dosis más altas de vacuna combinada. En el grupo vacunado con 0.1 mg de BCG no se observé adenitis supurativa. Los cultivos y la histopatología descarlaron las posibilidades de una infección progresiva por BCG o de una infection asociada. Considerando los resultados anteriores, se aceptô la combination tie 0.05 ing de BCG + 6 x 108 MLMC como la vacuna nuis apropiada para ensayos de canipo de amplia cobertura en el sur de la India.An effective antileprosy vaccine should protect against infection by Mycobacterium leprae or have therapeutic value against the disease. A number of potential vaccines based on either live BCG alone or with killed M. leprae (KML) or other killed mycobacteria such as ICRC, Mycobacterium w. and M. vaccae have been developed and have been claimed to be immunotherapeutic (2,4,18,20). Some of them are currently being evaluated for their immunoprophylactic efficacy against leprosy (6). The combination vaccine BCG plus KML was also tested for immunoprophylactic efficacy, and it was seen that there were 18% fewer cases from the vaccine group BCG plus KML than from the BCG group (3). But, no difference was seen between the BCG plus KML group and the BCG group among the general population in the Karonga Prevention Trial (11). Apart from being effective, a good vaccine needs to be safe with minimal side effects so that it is acceptable to the population using it. In Phase-II and extended Phase-II studies, three different population sets in Thiruthani taluk of Chengai-MGR District in Tamil Nadu, India, were tested during the period between August 1989 and October 1990 to ascertain the acceptability of the vaccines and the side effects, if any. In this communication, we are reporting on episodes of regional lymphadenitis in subjects who received BCG plus KML.
MATERIALS AND METHODS
Skin-test antigens and vaccines
The following biologicals were used in the study: a) BCG batch 308, June 1989 (viability count of 6.6 x 106 per ml) supplied by BCG Laboratory, Madras, India, as 0.1 mg per dose; b) armadillo-derived killed M. leprae Lot IV; c) Rees' M. leprae solubre skin test antigen (MLSA) Lot Wel-4-EFI as µ g protein per dose and d) lepromin-A Lot J-15-4, 21 7 88 as 30-40 million bacilli per ml supplied by IMMLEP. The doses of the various vaccines/placebos per 0.1 ml used for the Phase-II study were: a) BCG 0.1 mg + 6 x 108 KML; b) BCG 0.1 mg + 5 x 107 KML; c) BCG 0.1 mg + 5 x 106 KML; d) BCG 0.1 mg, or e) normal saline. For the extended Phase-II study they were: a) BCG 0.05 mg + 6 x 108 KML; b) BCG 0.05 mg + 5 x 107 KML; c) BCG 0.01 mg + 5 x 107 KML, or d) normal saline.
Subjects and follow up
In the Phase-II study, a total of 997 healthy volunteers (free from clinical leprosy and free from other contraindications for vaccines) in the age group 1-70 years were vaccinated on randomization with one of the four vaccines in 0.1 ml or with the control preparation. The study population included 247 children in the 1-6-year age group and 250 children in the 7-12-year age group; the remaining 500 individuals were in the 13-70-year age group. Vaccine was administered intradermally into the left deltoid region.
Further, the vaccinees were skin tested with Rees' MLSA on the upper third of the dorsum of the left forearm and with lepromin-A on the midvolar aspect of the right forearm at the time of vaccination and at 12 weeks post-vaccination. Readings were taken for induration to ascertain the sensitization potential of the vaccines. The vaccine site was examined at 3, 8, 12 and 15 weeks post-vaccination. Details have been published earlier (7,9).
In the extended Phase -ll study, in one group of villages, 860 healthy volunteers in the age group 1-70 years were vaccinated similarly with one of the three combination vaccines or placebo, skin tested with Rees" MLSA and lepromin-A at 12 weeks postvaccination, and readings were taken as in the Phase -ll study. In another village, 437 healthy subjects in the age group 13-70 years were vaccinated with BCG 0.1 mg in 0.1 ml. Details have been published earlier (9).
Follow up
All of the vaccinées were kept under surveillance to monitor for post-vaccination side effects, if any. In the Phase-II study, the study villages were visited once a week by a medical officer and vaccine-related complaints were recorded and followed up subsequently as detailed earlier (7,9).
In the extended Phase -ll study, the study subjects were visited on a weekly basis and specific enquiries were made for vaccinerelated side effects, particularly regional lymph node enlargement. Clinical findings were recorded every week until healing took place.
Laboratory methods
In the Phase -ll study, blood samples were collected from all vaccinées, and the serum was tested for phenolic glycolipid-I (PGL-I) antibody level by an ELISA and anti-35 kDa protein antibody by the serum antibody competition test (SACT).
In the extended Phase-II study, lymph node biopsies were obtained under local anesthesia and were divided into two parts. One part was processed for culture for acidfast bacilli (AFB) and also for non-AFB organisms. The other part was fixed in 10% formalin and routinely processed. Paraffinembedded sections were stained with a) hematoxylin and eosin and b) by the Fite-Farraco method for AFB. Further, the tissues were stained with anti-BCG antibody (DAKO Corporation, Copenhagen, Denmark) using the indirect immunoperoxidase method) (l2).
RESULTS
PhaseII
Out of 997 vaccinees, 13 individuals developed signs of regional suppurative lymphadenitis. All of these individuals were followed up clinically. Of these 13, 7 were in the group that received BCG 0.1 mg + 6 x 108 KML, 3 each in the two groups that received BCG 0.1 mg + 5 x 107 KML and BCG 0.1 mg + 5 x 106 KML. respectively. One subject in the group BCG 0.1 mg + 6 x 108 KML was in the age group 7-12 years; the remaining 12 were in the age group 13-70 years (Table 1).

The course of events leading to suppurative adenitis in all of the individuals was similar as judged by their histories and observations. Regional lymph node (axillary or supraclavicular) enlargement was fust noticed by these vaccinees around week 2, and it progressed to suppuration by week 8. Of these 13 cases, 7 were treated with incision and drainage; in the remaining 6 subjects the abscess opened and drained by itself. Except for cleaning and dressing with sterile dry gauze, no treatment was needed and recovery was uneventful.
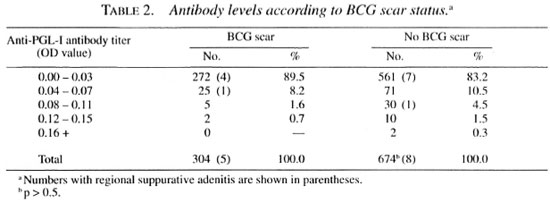
Previous BCG scar status and serology (antibodies against PGL-I and 35-kDa protein of M. leprae) did not reveal any helpful clues to identify the group at risk. It was interesting to note that the anti-PGL-I antibody and the anti-35-kDa protein antibody levels were extremely low in this population (Table 2). Out of 978 serum samples tested by ELISA, 833 (85.2%) showed anti-PGL-I antibody levels in the range of 0.00 to 0.03 OD units, 96 (9.8%) samples in the range of 0.04 to 0.07 OD units, and 49 (5.0%) in the range of 0.08 to 0.16 OD units. Among the 13 individuals with suppurative adenitis, 1 individual (14 years, BCG scar negative group) showed an antibody level of 0.09 OD units; 1 individual (38 years, BCG scar positive group), 0.04 OD units; all of the other 11 individuals, 0.00 OD units. Similarly, only 4 individuals (3 in BCG scar positive group and 1 in the BCG scar negative group) out of 978 samples tested were positive by SACT, and all of these four belonged to the 13-70-year age group. All 13 individuals with suppurative adenitis were negative by SACT. Suppurative adenitis was observed in 5 out of 304 vaccinées in the BCG scar positive group and in 8 out of 674 BCG scar negative group (p > 0.5). It was found that suppurative adenitis had a direct relationship to the age of the subjects and the dose of the antigens. Among 13 individuals with suppurative adenitis, 12 were from the 13-70 year age group; 6 of these 12 individuals were from the vaccinées who received the highest dose, BCG 0.1 mg + 6 x 108 KML. None of the individuals in the 1-6-year age group, even with highest dose, developed suppurative adenitis.
Extended Phase-II
BCG 0.1 mg was given to 437 subjects in the age group 13-70 years and two of them had an enlarged left axillary lymph node by week 2 after vaccination. By week 8, the lymph nodes were not palpable. No case of suppurative adenitis was observed in this group.
With the combination vaccine BCG plus KML, we observed four cases of suppurative regional lymphadenitis. Of the 200 individuals who were vaccinated with BCG 0.05 mg + 6 x 108 KML, eight developed transient regional lymphadenitis which did not require any treatment and subsided uneventfully by week 15. Two vaccinees developed suppurative adenitis; one male (age 9) had a left supraclavicular lymph node involvement and another male (age 13) presented with a left axillary lymph node involvement. The onset and sequence of events leading to suppuration were similar to the observations in the Phase-II study. Lymph node specimens from both of these cases subjected for culture were negative for both AFB and non-AFB organisms. Histologically, AFB and mycobacterial antigens could not be demonstrated in either case. However, epithelioid cells, fibroblasts, plasma cells and few poorly formed giant cells were seen. In addition, the supraclavicular lymph node showed small areas of necrosis with minimal karyorrhexis.
Two other cases were seen with the lower dose of combination vaccines. One male (age 40) from the vaccine group BCG 0.05 mg + 5 x 107 KML and one male child (age 12) from the BCG 0.01 mg + 5 x 107 KML group developed left axillary suppurative adenitis (Table 3). The onset of lymph node enlargement was seen by week 16 and week 18, respectively, and suppuration by weeks 18 and 20.
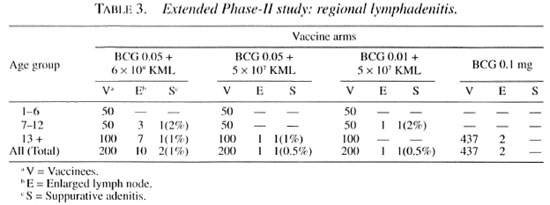
Histologically, these two cases resembled the adenitis seen in the patients given BCG 0.05 mg + 6 x 108 KML. However, in one of them (12-year-old male given BCG 0.01 mg + 5 x 107 KML) a minimal amount of mycobacterial antigen could be demonstrated. Culture from the biopsy material was negative for both AFB and non-AFB organisms. It was seen that 8 out of 12 individuals with lymph node enlargement were from the 13-70 -year age group receiving combination vaccines and 2 among this group developed suppurative adenitis.
Post-lepromin test regional adenitis
In addition to the above, two subjects developed regional adenitis following lepromin testing. Laboratory investigations were not performed on them. Their brief case histories are given below.
A female (age 38) who was vaccinated with BCG 0.1 mg + 6 x 108 KML in the Phase-II study had suppurative axillary lymphadenitis at 8 weeks post-vaccination. She was skin tested at 12 weeks post-vaccination with Rees' antigen and lepromin-A. A week later she developed fever and supratrochlear lymph node enlargement on the side of the lepromin test. This adenitis gradually subsided in 3 weeks.
In a 13-year-old female, who received BCG 0.01 mg + 5 x l07 KML. the vaccination lesion healed uneventfully without any regional lymph node enlargement, but she developed lymph node enlargement on the ipsilateral supratrochlear region 4 weeks after relesting with lepromin-A. The lymph node suppurated and drained itself within about 3 weeks, subsequently healing and leaving a healthy scar.
DISCUSSION
The occurrence of local lymphadenopathy following immunization is a wellknown phenomenon both in experimental animals and in man. Clinical enlargement of the local draining lymph node with BCG vaccination alone is reported to occur in 0.1% to 0.3% of the vaccinated individuals (1). Convit, et al. (2,3) observed suppurative adenitis in an extremely small number of individuals-only 4 in a total of about 30,000 vaccinees in Venezuela. In the Karonga prevention trial, reference was made to glandular abscesses and exceedingly large ulcers by the authors (15).
In our experience, in the Phase-II study, suppurative adenitis was observed mainly in the subjects belonging to the 13-70-year age group. This occurred in 6 out of 100 individuals receiving BCG 0.1 mg + 6 x 108 KML and 3 out of 100 in those receiving either BCG 0.1 mg + 5 x 107 KML or BCG 0.1 mg + 5 x 106' KML.
In the younger age groups, 1 out of 50 vaccinees (7-12 years) who received the highest dose (BCG 0.1 mg + 6 x 108 KML) developed suppurative adenitis, but other vaccinees in the 1-6-year age group and all of those in the 1-6- and 7-12-year age groups who received BCG 0.1 mg + 5 x I07 KML and BCG 0.1 mg + 5 X 106 KML, respectively, remained free from complication.
In the extended Phase-II study, the occurrence of suppurative adenitis could be reduced to 1% by lowering the BCG dose alone to half, i.e., in the vaccine arm BCG 0.05 mg + 6 X 108 KML. However, further lowering of the BCG and KML dose did not result in totally overcoming the occurrence of suppurative adenitis (Table 3). It was interesting to note that in individuals receiving a lowered dose, BCG 0.05 + 5 X L07 KML and BCG 0.01 mg + 5 X 107 KML, lymph node enlargement was seen by weeks 16 and 18 post-vaccination and suppuration by about weeks 18 and 20, while with BCG 0.05 mg + 6 X 108 KML the suppuration occurred in two individuals by week 8.
Since the mycobacteria could not be grown in culture, it was clear that the adenitis was not due to progressive infection caused by BCG. Similarly, the biopsied material did not show the presence of nonacid-fast organisms, thus ruling out the possibility of bacterial contamination of the vaccine or superadded infection. The staining of tissues with antimycobacterial antibody using the immunoperoxidase technique is quite sensitive, and this method has been utilized to detect the presence of mycobacterial antigen in both human (12) and animal (14) tissues. Further, we used anti-BCG antibody since this has been shown to detect antigens which are common to both BCG and k leprae (l0). The absence of AFB in all of the cases and the minimal presence of mycobacterial antigen in only one case in the tissue indicated that the biopsies were probably performed in the resolving stage. Uneventful healing and subsidence of the lymph node swelling without any specific intervention measures would seem to support such a view.
Narayanan and his colleagues chartered the course of regional lymph node involvement following BCG, M. leprae, or M. kansasii vaccination in the guinea pig (13). They found that while BCG and M. kansasii induced maximum granuloma in the draining lymph node in 2-3 weeks, M. leprae took nearly 5 weeks to induce a granuloma. These granulomas almost completely resolved in about 10 weeks. In human beings, the development of lymph node abscess following BCG is very uncommon and virtually restricted to the lirst year of life, with local lymph node softening (16). Among children, the occurrence of suppurative adenitis may be due to deeper vaccination, higher dose of BCG, or a highly potent BCG strain (5,19). In adults, one incident of local and regional lymph node abscess following a large overdose about 40 times higher than the normal dose had been recorded (21).
The present report is the first on the BCG and KML combination causing suppurative adenitis. However, in the present study, since none of the children in the 1-6-year age group developed suppurative adenitis and, moreover, in adults the course of events was also uneventful after drainage and did not require any therapy, the possibilities of deeper vaccination and a large overdose can be ruled out. The different time courses of lymphadenitis observed in the vaccinated individuals reported here could be related to a number of factors, such as the dose of the organisms, prior exposure of the individuals to mycobacteria and their innate ability to mount a hypersensitivity response. Earlier a high level of nonspecific sensitization to mycobacterial antigens had been demonstrated among the population of Chengalpattu District (8).
The precise mechanisms involved in the development of suppurative adenitis are not clear. Skin-test reaction to Rees' antigen and lepromin-A did not show any association with regional lymphadenitis (7). Although the levels of antibody against M. leprae in the serum were not elevated, one feature worthy of note in these cases was the large number of plasma cells observed in the biopsies from three subjects. The role of these cells remains speculative although antibodies have been implicated in the genesis of some forms of mycobacterial granuloma (l7).
It is possible that high levels of specific and nonspecific mycobacterial sensitization are responsible for the occurrence of adenitis when the dose of the antigen is increased, especially in subjects belonging to the older age groups. None of the 636 subjects who received only BCG 0.1 mg developed suppurative adenitis. By merely reducing the BCG dose to half, the rate of suppurative adenitis was reduced dramatically to a minimal self-limiting process.
Adenitis appears to be more a function of total antigen load than any direct BCG effect. In view of the foregoing, it was decided that BCG 0.05 mg + 6 x 108 KML could be accepted for the large-scale vaccine trial in South India.
Acknowledgment. Our sincere thanks are due to the vaccinées who volunteered and cooperated with us during the entire follow-up period. The supply of vaccine grade killed M. leprae, lepromin-A, and Kees' antigen from the IMMLEP/WHO Special Programme for Research and Training in Tropical Diseases (TDK) is gratefully acknowledged. The authors extend their sincere thanks to Dr. C.N. Paramasivan, former Officer-in-Charge. Tuberculosis Research Centre (IRC), Madras, India, for the support to carry out the culture methods at IRC, Madras. Anti-PGL-I LLISA and SACT for the 35-kDa protein were undertaken in the immunology laboratory at CJIL, Agra, India, and we are grateful to Dr. U. Sengupta, Director, CJIL, Agra, for the same. Thanks are due to Mr. S. Kannan, ARC), for assistance in compiling and analyzing the data. We thank Mr. K. Arumugam for secretarial assistance.
REFERENCES
1. BHUSHAN, K. Normal and abnormal BCG reactions. NT1 Newsletter 15(1978)65-69.
2. CONVIT, J., ARANZAZU, N., ZUNIGA, M., ULRICH, M., PINARDI, M. L., CASTELLAZZI, Z. and ALVARADO). J. Immunotherapy and immunoprophylaxis. Lepr. Rev. 54 Special Issue (1983) 47s-60s.
3. CONVIT, J., SAMPSON, C, ZUNIGA, M., SMITH, P. G., PLATA, J., SILVA, J., MOLINA, J., PINARDI, M. E., BLOOM, B. R. and SALGADO, S. Immunoprophylactic trial with combined Mycobacterium leprae BCG vaccine against leprosy: preliminary results. Lancet 339(1992)446-450.
4. DEO, M. G., BAPAT, C. V., BHALE RAO, V., CHATURVEDI, R. M., CHULAWALA, R. G. and BHATKI, W. S. Potential antileprosy vaccine from killed ICRC bacilli-a clinicopathological study. Indian J. Med. Res. 74(1981) 164-171.
5. GRIFFITH, A. H. Overdose of BCG vaccine. Tubercle 44(1963) 247.
6. GUPTE, M. D. Vaccines against leprosy. Indian J. Lepr. 63(1991)342-349.
7. GUPTE, M. D., ANANTHARAMAN, D. S., BRITTO, L. J., VALLISHAYEE, R. S., NAGARAJU, B., KANNAN, S. and SENGUPTA, U. Sensitization potential and reactogenicity of BCG with and without various doses of killed M. leprae. Int. J. Lepr. 60(1992)340-352.
8. GUPTE, M. D., ANANTHARAMAN, D. S., NAGARAJU, B., KANNAN., S. and VALLISHAYEE, R. S. Experiences with Mycobacterium leprae soluble antigens in a leprosy endemic population. Lepr. Rev. 61(1990)132-144.
9. GUPTE, M. D., VALLISHAYEE, R. S., BRITTO, L. J. and ANANTHARAMAN, D. S. Sensitization potential and reactogenicity of varying doses of BCG plus killed Mycobacterium leprae; an extended study. Int. J. Lepr. 61(1993)563-569.
10. HARBOE, M, MSHANA, R. N. CLOSS, O., KRONVALL., G. and AXELSEN, N. H. Crossreactions between mycobacteria-II. Crossed inmiunoelectrophoretic analysis of soluble antigens of BCG and comparison with other mycobacteria. Scand. Immunol. 9(1979)115-124.
11. KARONGA PREVENTION TRIAL GROUP. Randomized controlled trial of single BCG, repeated BCG, or combined BCG and killed Mycobacterium leprae vaccine for prevention of leprosy and tuberculosis in Malawi. Lancet 348(1996)17-24.
12. MSHANA, R. N., BELEHU, A., STONER, G. L., HARBOE, M. and HAREGEWOIN, A. Demonstration of mycobacterial antigens in leprosy tissues. Int. J. Lepr. 50(1982)1-10.
13. NARAYANAN, R. B.; BADENOCH-JONES, P. and TURK, J. L. Experimental mycobacterial granulomas in guinea pig lymph nodes: ultrastructural observations. J. Pathol. 134(1981)253-265.
14. ORELL, J. M., BRETT, S. J., IVANYI, J., COGHILL, G., GRANT, A. and BECK, J. S. Measurement of the tissue distribution of imnumoperoxidase staining with polyclonal anti-BCG serum in lung granulomata of mice infected with Mycobacterium tuberculosis. J Pathol. 164(1991)41-45.
15. PONNIGHAUS, J. M., FINE., P. E. M., BLISS, L., GRUER, P. J. K., KAPIRA-MWAMONDWE, B., MSOSA, E., REES, R. J. W., CLAYTON, D., PIKE, M. C, STERNE, J. A. C. and OXBORROW, S. M. The Karonga prevention trial: a leprosy and tuberculosis vaccine trial in northern Malawi. I. Methods of the vaccination phase. Lepr. Rev. 64(1993)338-355.
16. SlMMONDS, F. A. H. Delayed regional adenitis after BCG vaccination. Tubercle 45(1964)160.
17. SPECTOR, W. G., MARIYANAYAGAM, Y. and RIDLEY, M. J. The role of antibody in primary and reinfection BCG granulomas of rat skin. J. Pathol. 136(1982)41-57.
18. TALWAR, G. P., ZAHEER, S. A. and MUKHERJEE, R. Immunotherapeutic effects of a vaccine based on a saprophytic cultivable mycobacterium, Mycobacterium, w. in multibacillary patients. Vaccine 8(1991)121-129.
19. TEN DAM , H. G., TOMAN, K., HITZE, K. L. and GULD, J. Present knowledge of immunization against tuberculosis. Bull. WHO 54(1976)255-269.
20. WALIA, R., SARATHCHANDRA, K. G., PANDEY, R. M., PARIDA, S. K., ZAHEER, S. A., KAR, H. K., MUKHERJEE, A., MUKHERJEE, R. and TALWAR, G. P. Field trials on the use of Mycobacterium w. vaccine in conjunction with multidrug therapy in leprosy patients for immunotherapeautic and immunoprophylaxis purposes. Lepr. Rev. 64(1993)302-311.
21. WATKINS, S. M. Unusual complications of BCG vaccination. Br. Med. J. 1(1971)442.
1. M.D.
2. M.D., D.P.H., Officer in Charge, CJIL Field Unit (Indian Council of Medical Research), 271 Nehru Bazaar. Avadi, Madras 600 0.54. India.
3. M.B.B.S., Ph.D., Tuberculosis Research Centre (Indian Council of Medical Research), Spurtank Road, Madras 600 031, India.
Reprint requests to Dr. Gupte.
Received for publication on 17 June 1996: accepted for publication in revised form on 2 December 1996.