- Volume 65 , Number 1
- Page: 20–7
Effect of steroid therapy on parameters of peripheral autonomic dysfunction in leprosy patients with acute neuritis
ABSTRACT
Recent electrophysiological studies on peripheral autonomic dysfunction in leprosy patients show a high prevalence of autonomic dysfunction as measured by abnormal vasomotor reflexes (VMR) and absent sympathetic skin response (SSR). Nothing is known about the reversibility of these autonomic parameters with treatment. Since there is evidence that small fiber function may be the most reversible component in neuropathies, we measured the effect of steroid treatment on autonomic parameters together with motor and sensory functions in leprosy patients with acute neuritis. Control subjects were investigated for repeatability testing of autonomic function. Due to a relatively high variability on repeat VMR testing in the controls, we defined a change in VMR testing as a change of > 30%. With this definition, the VMR of 14.8% of the patients improved, 75% remained unchanged, and 10.2% worsened. Absent SSR became positive in 16.6% and remained unchanged in 83.4%. Improvement in sensory and motor functions was seen in 21.2% and 1.3% of the patients, respectively.RÉSUMÉ
Des études électro-physiologiques recentes sur les dysfonctionnements autonomiques périphériques che/, les malades de la lèpre ont montré une prévalence élevée de dysfonctionnement autonomique tel que mesuré par des réllexes vasomoteurs (RVM) anormaux et l'absence de réponse sympathique cutanée (RSC). On ne sait rien de la réversibilité de ces paramètres autonomiques avec le traitement. Puisqu 'il y a des évidences que la fonction de la petite libre pourrait être la composante la plus réversible dans les neuropathies, nous avons mesuré l'effet du traitement stéroïdien sur les paramètres autonomiques, ainsi que les fonctions nerveuses et sensorielles chez, des malades de la lèpre présentant une névrite aiguë. On a examiné des sujets-témoins quant à la reproductibilité des tests de fonction autonomique. Du fait d'une variabilité relativement élevée des tests répétés de RVM chez les témoins, nous avons défini un changement dans un test de RVM comme étant un changement supérieur à 30%. Avec cette définition, le RVM s'est amélioré chez 14.8 % des patients, est resté inchangé chez 75%, et a empiré chez 10.2%. Une RSC absente devint positive dans 16.6% et resta inchangée dans 83.4%. Une amélioration dans les fonctions sensitive et motrice a été observée dans respectivement 21.2% et 1.3% des patients.RESUMEN
Algunos estudios electrofisiológicos recientes sobre la disfunción periférica autónoma en los pacientes con lepra mostraron una alta prevalencia de disfunción autónoma (rellejos vasomotores, RVtVl, anormales) y ausencia de respuesta simpática de la piel ( RSP). No se sabe nada sobre la reversibilidad de estos parámetros de función autónoma en relación al tratamiento. Puesto que hay evidencias de que la función de las pequeñas fibras, es el componente más reversible en las neuropatías, en este estudio medimos el efecto del tratamiento con esteroides sobre los parámetros autónomos y las funciones motora y sensorial en los pacientes con lepra y neuritis aguda. Se incluyeron sujetos control para investigar la función autónoma. Debido a que las pruebas sobre los RVM aplicadas a los controles resultaron muy variables, establecimos que hubieron cambios en los RVM cuando estos cambios fueron superiores al 30%. Con esta definición, encontramos que como resultado del tratamiento, los RVM de 14.8% de los pacientes mejoraron, en 75% permanecieron sin cambio, y en 10.2% empeoraron. Las pruebas sobre la RSP llegaron a ser positivias en 16.6% de los pacientes y permanecieron sin cambio en el 83.4%. La mejoría en las funciones sensorial y motora se observó en el 21.2 (sensorial) y en el 1.3% (motora) de los pacientes.Nerve damage is the major cause of longterm disability in leprosy (6,11). Consequently, the primary prevention of longterm disabilities in leprosy will only be achieved by early detection and adequate treatment of neural impairment. The benefits of steroid treatment of leprosy reactions and neuropathy have been recognized for several decades (18). Studies assessing the outcome of antineuritis treatment have used motor and/or sensory functions as parameters (13,25,27).
The effect of steroid therapy on the parameters of autonomic function is unknown. Since autonomic nerve dysfunction may be the earliest and most reversible component of neuropathies (8), sequential electrophysiological studies of autonomic function may prove important for monitoring the treatment of neuropathy in leprosy.
Recent studies on peripheral dysautonomia in leprosy were based on vasomotor reflex (VMR) testing (1,3,29,30) and the sympathetic skin response (SSR) (28,30). VMR measures the reduction in fingertip skin blood flow in response to a test stimulus and is an index of peripheral autonomic function (14,l5). SSR is a simple autonomic test measuring the changes in skin voltage in response to exosomatic stimuli with sweat glands as effectors (2,16,19,23). A high prevalence of impaired VMR and SSR was found in leprosy patients (1,3,29). It remains to be established whether these lesions are permanent or reversible.
The aims of this pilot study were to describe the short-term effects of steroid treatment on two parameters of peripheral autonomic function (VMR and SSR) in comparison with motor and sensory nerve functions.
STUDY PARTICIPANTS AND METHODS
Patients
A prospective pilot study was carried out at the Green Pastures Hospital (GPH) in Pokhara, West Nepal, between April and June 1995. All leprosy patients [confirmed according to World Health Organization (WHO) criteria] attending the GPH neuritis clinic were included in the study if they fulfilled the inclusion criteria: age range between 10 and 55 years, voluntary participation after informed consent, current treatment with standard WHO-recommended multidrug therapy (WHO/MDT), and a course of steroid treatment for neuritis due to leprosy. The diagnosis of neuritis was made by three experienced medical doctors at the neuritis clinic and was based on the following criteria: a) Skin = redness and swelling of skin lesions and tenderness of the lesions, b) Peripheral nerves = swelling on palpation, and/or spontaneous pain, and/or tenderness on palpation, and/or paresthesia and/or nerve function impairment [as tested by touch sensibility test (TST) and voluntary muscle test (VMT), see below), c) General = sometimes edema of hands, feet or face, occasionally fever.
The skin signs were obligatory, the nerve signs and general signs optional. Patients with erythema nodosum leprosum (ENL) were not included.
Corticosteroid treatment was given in the following way: Prednisolone 40 mg initially, tapering approximately 5 mg every 2 weeks, depending on the progress of the patient as judged by serial TST and VMT measurements. Depending on the clinical severity of neuritis and steroid side effects, individual dose adjustments could be undertaken.
Exclusion criteria were ulcers on or reabsorption of more than one fingertip, more than one missing digit, and patients suffering from alcoholism or diabetes mellitus.
Controls
Healthy Nepali volunteers between the ages of 10 and 55 were recruited from the Pokhara Red Cross Organization. In addition, some of the Nepali hospital staff recruited friends. All controls had no known contact with leprosy patients. All patients and controls were recruited by an independent investigator.
The study was approved by the local ethical committee in Berne, Switzerland, and in Pokhara, Nepal.
Measurement of autonomic reflexes
All subjects were allowed to equilibrate at an ambient temperature of 28-34°C in a quiet room. The procedure was explained to them in Nepali by a translator. The neurophysiological tests were performed by a consultant in neurology or were performed under his direct supervision. Repeat examinations were conducted at an interval of between 10 to 40 days for leprosy patients and 3 to 40 days for controls.
Vasomotor reflex (VMR). A laser-Doppler flow-temperature monitor (Model DRT4; Moor Instruments, Axminster, England) with machine settings of band width 10 KHz, time constant 1 sec, gain control and zeroing automatic was used to measure fingertip blood How. Low, el al. 's guidelines (15) were used. Double-sided adhesive disk secured attachment of the combined laser-Doppler to the skin. Fingertip blood flow was taken as the mean of the laser-Doppler measured red blood cell flux value during a 3-min observation period. An inspiratory gasp was used to induce the VMR. Subjects were asked to practice to "take the quickest and deepest breath you can and hold it for 10 seconds." The largest response elicited from three gasps was used for subsequent analysis. For each subject, 12 recordings were made (10 fingers, 2 big toes). The onset of each inspiratory gasp was marked with an event marker, and the resultant maximal reduction in skin blood flow recorded and expressed as a percentage of the resting skin blood flow. VMR testing was not begun until the inspiratory gasp was satisfactorily mastered and a stable baseline blood flow was recorded. A more complete description of VMR testing is presented elsewhere (15-29).
The definition of an abnormal VMR [modified criteria of Low, et al. (15)] was based on percentage reduction of blood flow below the 5th percentile. To define the hand as abnormal, more than 1 of the 5 measurement sites had to be abnormal. The 5th percentile based on our previous study (28) was 39% for the digits of the hands. For the foot, we only measured the great toe; the 5th percentile was 25%.
Sympathetic skin response (SSR). The SSR was performed as previously described (23). Briefly, surface electrodes were attached to the palm and dorsum of either hand as well as to the sole and dorsum of either foot after cleaning with an alcoholic solution. Single square pulses of 200 msec were delivered to the skin of the wrist and ankle. If the skin in those areas was anesthetic, the pulses were applied to a sensitive skin area more proximally. The response was considered absent if no consistent voltage change using a sensitivity of 50 mV/cm was observed after at least 10 trials separated by long intervals to avoid the natural habituation of the response.
Touch sensibility test (TST). A standard set of five Semmes-Weinstein monofilaments was used as described by Bell-Krotoski (4). The score per site varies from 0-5. A score of 5 was given when the thinnest monofilament in the test series was felt (on the hand, 50 mg; on the feet, 200 mg); a score of zero if the thickest filament was not felt. These filaments give a force ranging from 50 mg to 300 mg for the hand and 200 mg to 300 mg for the feet when applied with enough force to bend the filament. The following sites were tested: 1) ulnar nerve: 3 points, on the pulp of the little linger, on the volar skin over the 5th metacarpophalangeal (MCP) joint, and on the hypothenar eminence (maximum score 15); 2) median nerve: 3 points, on the pulp of the thumb, on the first MCP joint, and on the thenar eminence (maximum score 15); 3) posterior tibial nerve: 4 points, on the tip of the 5th toe, on the plantar skin over the 5th metatarsophalangeal joints, the lateral border and the heel (maximum 20). If there was an ulcer on the test site a score of zero was given for that site.
The maximum score of the TST was 100 for all sites together: 90-98 was defined as mild, 60-89 as moderate, and below 60 as severe sensory deficit.
Voluntary muscle test (VMT). The VMT was performed using the modified Medical Research Council (MRC) scale as described by Brandsma (6). The VMT score consisted of the sum (0-5) of individual scores (0 = paralyzed; 5 = normal strength) for muscles innervated by the ulnar nerve (first dorsal interosseus muscle and abductor digiti minimi, maximum score 10); median nerve (abductor pollicis brevis and opponens pollicis, maximum score 10); radial nerve (extensor carpi ulnaris and extensor digitorum communis, maximum score 10); lateral popliteal (common peroneal) nerve (extensor hallucis longus and peroncus longus and brevis; maximum score 10). Maximum score for all sites was 80. A combined score of 70-78 was delined as mild, 50-69 as moderate, and below 50 as severe motor deficit.
Improvement in VMT or TST score was defined as an increase of > 1 point, unchanged as a change in score between -1 and 1, and worsening as deterioration of > 1 point.
Statistical methods
The statistical packages EPI INFO Version 6 (Centers for Disease Control and Prevention, Atlanta, Georgia, U.S.A.) and SAS Version 6.11 (SAS Inc., Cary, North Carolina, U.S.A.) were used for data entry and statistical analysis. Repeatability of test results in controls was assessed by comparing values in paired / tests by plotting the differences between the two measurements against the mean of the two measurements. The chi-squared test was used for comparison of changes in the VMR and SSR in the controls versus the leprosy patients.
RESULTS
Eighteen patients with a mean age of 35.5 years (range 13-55, 14 males and 4 females) were included in the study: 9 patients were newly diagnosed with leprosy, 13 patients (including the 9 newly diagnosed) were started on steroid therapy at the time of our initial examination; the other 5 had been already on treatment for 1-6 weeks. Sixteen patients had multibacillary (MB) leprosy, two had paucibacillary (PB) leprosy. The mean age of the 17 controls was 32 years (range 11-55, 10 males and 7 females). The mean interval time for repeat examination in the patient group was 21.3 days (range 10-40 days); in the control group, 10 days (range 3-21 days).
Initial examination
Controls. VMR was abnormal in 5 of 68 limbs (7.3%), SSR in 2 out of 68 limbs (2.9%). The mean TST score was 96 (maximum 100). All TST measurements were within the normal range since no subject scored more than 1 point below the maximum score per tested sensory nerve. The VMT score was normal (80 out of 80).
Leprosy patients. Thirty-seven out of 72 limbs were abnormal (51.2%) in VMR testing. Absent SSR were recorded in 55 out of 72 limbs (76.4%). All 18 leprosy patients had sensory deficits, 17 patients had additional motor deficits. Seven patients had mild, 6 moderate and 5 severe sensory impairments. Ten patients had mild, 7 moderate and 1 severe motor nerve impairments. The mean TST score was 75 (maximum 100); for VMT 73 (maximum 80). The results of the initial examination are summarized in Table 1.
Repeatability of autonomic tests in controls
VMR. The mean absolute differences between the two measurements were between -7 % and 4% blood flow reduction, and probability values calculated from paired / tests were between 0.14 and 0.8.
Thus, there was no evidence of systematic error. However, plots of differences against the mean of the two measurements show considerable within-person variability, with repeatability coefficients ranging from 23% to 47%. In view of this considerable withinperson variability, the cut-off level separating pathological from normal fluctuation needs special consideration. We tested two different cut-off values: a) > 20% change of VMR, difference; b) > 30% change of VMR. Table 2 presents the results. The > 30% change of VMR was chosen since with this controls remained within the statistically expected range. We then used the > 30% change of VMR as the definition for a change in leprosy patients. An increase of > 30% of VMR was interpreted as "improvement," a change of VMR < 30% as "unchanged," and a decrease > 30% of VMR as "worsening." Accordingly, 91.3% remained unchanged, 5.3% improved and 3.4% worsened.
SSR. The SSR remained unchanged in 91.3%; 5.8% of initially present SSR were absent on repeat testing; 2.9% of initially absent SSR became positive on repeat testing.
TST/VMT. No changes of more than 1 point were observed in controls.
Repeat examination after initiation of steroid therapy in leprosy patients
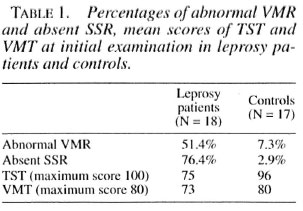
VMR. Using a cut-off value of > 30% change in the VMR, 14.8% of the leprosy patients improved, 75% remained the same, and 10.2% deteriorated. The changes in VMR in leprosy patients compared with the repeat testing in controls were highly significant (p < 0.001) (Fig. 1).
Fig . 1. Comparison of changes in VMR measurements between controls and patients. Improvement is defined as an increase in VNIR by > 30%, worsening as a decrease by > 30%, with unchanged ranging in between. Chi-squared test between the two groups is p <0.001. ■ = Worsening;  unchanged;
unchanged;  = improvement.
= improvement.
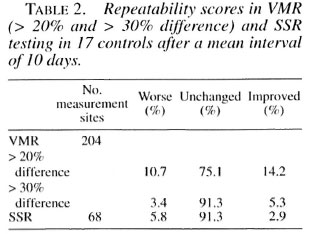
SSR. The 16.6% initially absent SSR became positive on repeat examination; 83.4% remained unchanged. No initially present SSR changed to absent (worsened) on repeat testing (Fig. 2). The changes for SSR were highly significant (p < 0.001) compared with controls.
Fig . 2. Comparison of changes in SSR between controls and patients. Improvement is defined as aninitially absent response becoming present on repeat testing; worsening as an initially present response becoming absent. Chi-squared test between the two groups is p < 0.001.  = Improvement;
= Improvement;  = unchanged; ■ = worsening.
= unchanged; ■ = worsening.
Correlation VMR and SSR. Based on the definition of an abnormal hand for VMR testing (more than 1 digit had to have a blood llow reduction below 39%), we compared the hands separately for parallel changes (i.e., improvement, deterioration or no change) for VMR and SSR. With the right hand 13 out of 18 showed parallel changes. In the left hand 50% (9 out of 18) showed parallel changes. Our patients duration of leprosy varied between a new diagnosis and 120 months. Because of the small numbers we were not able to group according to the duration of neuritis. Overall, there is no correlation between the two autonomic parameters for VMR and SSR.
TST. Sensory function improved in 21.2%, remained the same in 70.3% and worsened in 8.3%.
VMT. Motor function improved in 1.3%, remained the same in 96.5% and worsened in 2%. Thirteen patients started on steroid treatment at initial examination showed no significant differences for VMR, SSR, TST and VMT compared to all 18 patients (data not shown). Table 3 and Figure 3 summarize the results of changes in the VMR, SSR, TST and VMT testing in leprosy patients.
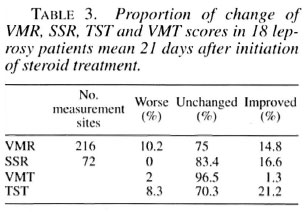
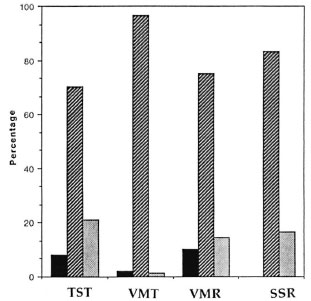
Fig. 3. Comparison of changes in TST, VMT,VMR, and SSR after initiation of steroid treatment for acute neuritis in leprosy patients. TST and VMT: Improvement in VMT or TST score was defined as an increase of > 1 point; unchanged as a change in score between - 1 and 1; worsening as deterioration of > 1 point.VMR: Improvement is defined as an increase in VMR by > 30%; worsening as a decrease by > 30%; unchanged ranging in between. SSR: Improvement is de-fined as an initially absent response becoming present on repeat testing; worsening as an initially present response becoming absent.  = Improvement;
= Improvement;  = unchanged; ■ = worsening.
= unchanged; ■ = worsening.
DISCUSSION
The benefits of steroid treatment on sensory and motor impairments in neuropathy in leprosy are well documented (18,26). The effect of steroid therapy on autonomic dysfunction in leprous neuropathy is hitherto unknown. Our results suggest that steroid treatment for leprosy neuritis can have a positive effect on autonomic function as tested by two independent parameters (the VMR and SSR). However, as with sensory and motor impairments, a large proportion of patients show no improvement at all. Deterioration of autonomic function was only recorded for VMR in a small proportion of leprosy patients. No deterioration of the SSR was documented.
Our pilot study cannot conclusively determine the changes in autonomic function in leprosy patients as the result of steroid treatment, since this would require a placebo-controlled study which is ethically not sustainable.
The larger proportion of sensory over motor function improvement agrees well with a previous study performed at the same hospital (26). However, the proportion of improvement in our study was much smaller for both parameters despite tests being performed by the same examiners using the same tests. Most likely this difference is due to our shorter interval time of repeat testing, further confounded by a smaller patient number.
Time-trend studies report the most rapid improvement occurring during the first month of steroid treatment with most improvement during the first 2 weeks (17,26). In the first case report on sequential electrophysiological autonomic tests in a leprosy patient on steroid treatment for acute neuritis, significant changes were described within the first 14 days (30). For these reasons a relatively short time span for repeat testing was used. In that case report a discrepancy between sequential SSR and VMR was noticed, with SSR improving but VMR deteriorating over time. Our present study also shows little correlation between these two autonomic parameters, although our patient population is too heterogenous (duration of leprosy varied between 0-120 months) and too small to allow any firm conclusions to be drawn at this stage. Both reflexes have complex mechanisms (14), their end organ response being unmyelinated sympathetic fibers with two different components. The vasomotor component for VMR and the sudomotor component for SSR are capable of independent responsivity to different stimuli (2). It is conceivable that the damage to nerve endings innervating smooth muscle in the vessels compared to those innervating sweat glands might follow a different time course with consecutive differing nerve recovery responses to steroid treatment.
Previous studies on the effect of steroid treatment in neural impairment in leprosy have been based mainly on subjective measurements of nerve function, such as the TST and VMT. More objective test methods of a quantitative nature could, therefore, offer some advantage over clinical tests. Although this study was primarily aimed at the evaluation of the effect of steroid treatment on two autonomic parameters, further studies are needed to evaluate the potential of VMR and SSR testing in monitoring neuropathy in leprosy. One of the difficulties with VMR is its relatively high intra-person variability on repeat test(15,29), probably due to skin sympathetic fiber responsiveness to thermal and emotional stimulation (9). Therefore, we chose a cut-off level (30%) which generates the statistically expected levels of normal variation in controls. As a trade off, this relatively high cut-off level reduces the test sensitivity for detecting both improvement and deterioration. The high variability in VMR testing might, however, limit its subsequent use in sequential monitoring of autonomic nerve damage during clinical trials.
The main advantage of autonomic testing lies in its potential use in detecting early nerve damage in leprosy neuritis since there is ultrastructural (20,22), immunocytochemical (l2), and neurophysiological (1,3,29) evidence that autonomic fibers are affected earliest in leprosy neuropathy. Since corticosteroid treatment is known to be most effective in the recent onset of neuritis (24), early detection is crucial for the prevention of disabilities, thus justifying more sensitive test equipment compared to conventional tests such as the VMT or TST.
Our results suggest that autonomic nerve damage in leprosy is not necessarily a permanent lesion and may either improve with therapy or deteriorate as a sign of ongoing nerve damage. Further studies are required to investigate the long-term effect of steroid treatment on autonomic function in leprosy.
Acknowledgment. The authors wish to acknowledge the valuable statistieal help of Jo Morris, biostatistician at the London Insitute of Tropical Medicine and Hygiène. We are grateful for the financia! support by the Swiss Academy of Medical Sciences and Sandoz. We are indebted to the staff of the physiotherapy department at CPU for their valuable practical help. Without the support of Dr. W. van Brakel, (project manager of the Leprosy Control Project, Pokhara, Nepal), Allison Anderson, Ph.D., (biostatistician INF), ami Dr. I'rauke Worpel, (Superintendent of CPU) this work would not have been possible. The work ol'GPH for leprosy patients was initiated following Jesus Christ's example of Mark 1,41: "Jesus, moved with compassion, put out His hand and touched the leper."
REFERENCES
1. ABBOT, N. C, BECK, J. S., SAMSON, P. D., BUTLIN, C. R., BROWN, A. R., FORSTER, A., GRANGE, J. M. and CREE, I. A. Impairment of fingertip vasomotor reflexes in leprosy patients and apparently healthy contacts. Int. J. Lepr. 59(1991)537-547.
2. ARUNODAYA, C. R. and TALY, A. B. Sympathetic skin response: a decade later. J. Neurol. Sci. 129(1995)81-89.
3. BECK, J. S., ABBOT, N. C, SAMSON, P. D., BUTLIN, C. R., GRANGE, J. M., CREE, I. A., FORSTER, A. and KHAN, F. Impairment of vasomotor reflexes in the fingertips of leprosy patients. J. Neurol. Neurosurg. Psych. 54(1991)965-971.
4. BELL-KROTOSKI, J. A. "Pocket" filaments and specifications for Semmes-Weinstein monofilaments. J. Hand Ther. 3(1990)26-31.
5. BLAND, M. and AI.TMAN, D. G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1(1986)307-310.
6. BRANDSMA, W. Basic nerve function assessment in leprosy patients. Lepr. Rev. 52(1981)161-170.
7. BRYCESON, A. and PFALTZGRAFF, R. E. Leprosy. 3rd edn. Edinburgh: Churchill Livingstone, 1990, p. 127.
8. GREENE, D. A., BROWN, M. J., BRAUNSTEIN, S. N., SCHWARTZ, S. S., ASBURY, A. K. and WINLGARD, A. Comparison of clinical course and sequential electrophysiological tests in diabetics with symptomatic polyneuropathy and its implications for clinical trial. Diabetes 30(1981)139-147.
9. HAGBARTH, K.-E., HONGELL, A., TOREBJORK, H. E. and WALLIIN, B. G. General characteristics of sympathetic activity in human skin nerves. Acta Physiol. Scand. 92(1974)303-317.
10. HREIDARSSON, A. B. Acute reversible autonomic nervous system abnormalities in juvenile insulindependent diabetes: a puppillographic study. Diabetologia 20(1981)475-481.
11. JOB, C. K. Nerve damage in leprosy. Int. J. Lepr. 57(1989)532-539.
12. KARANTH, S. S., SPRINGALL, D. R., LUCAS, S., LEVY, D., ASHBY, P., LEVENE, M. M. and POLAK, J. M. Changes in nerves and neuropeptides in skin from 100 leprosy patients investigated by immunocytochemistry. J. Pathol. 157(1989)15-26.
13. KIKAN, K. U., STANLEY, J. N. A. and PEARSON, J. M. H. The outpatient treatment of nerve damage in patients with borderline leprosy using a semistandardized steroid regimen. Lepr. Rev. 56(1988)127-134.
14. Low, P. A. Autonomic nervous system function. J. Clin. Neurophysiol. 10(1993)14-27.
15. Low, P. A., NEUMANN, C, DYCK, P. J., FEALEY, R. D. and TUCK, R. R. Evaluation of skin vasomotor reflexes by using laser Doppler velocimetry. May Clin. Proc. 58(1983)583-592.
16. MASELLI, R. A., JASPAN, J. B., SOLIVEN, B. C, GREEN, A. J., SPIRE, J.-P. and ARNASON, B. G. W. Comparison of sympathetic skin response with quantitative sudomotor axon reflex test in diabetic neuropathy. Muscle Nerve 12(1989)420-423.
17. NAAFS, B. and DAGNE, T. Sensory testing: a sensitive method in the follow up of nerve involvement. Int. J. Lepr. 45(1977)364-368.
18. PEARSON, J. M. H. The use of corticosteroids in leprosy. Lepr. Rev. 52(1981)293-298.
19. SHAHANI, B. T, HALPERIN, J. J., BOULU, P. and COHEN, J. Sympathetic skin response: a method of assessing unmyelinated axon dysfunction in peripheral neuropathies. J. Neurol. Neurosurg. Psych. 47(1984)536-542.
20. SHETTY, V. P., ANTIA, N. H. and JACOBS, J. M. The pathology of early leprous neuropathy. J. Neurol. Sci. 88(1988)115-131.
21. SHITTY, V. P., MEHTA, L. N. and ANTIA, N. H. Unmyelinated fibres in leprosy neuritis; an ultrastructural study. Bull. Elec. Microsc. Soc. India 2(1978)2-5.
22. SHITTY, V. P., MEHTA, L. N., ANTIA, N. H. and IRANI, P. F. Teased fibre study of early nerve lesions in leprosy and contacts, with electrophysiological correlates. J. Neurol. Neurosurg. Psych. 40(1977)708-711.
23. SOLIVEN, B. C, MASELLI, R. A., JASPAN, J. B., GREEN, A. J., GRAZIANO, H., PETERSEN, M. and SPIRE, J. P. Sympathetic skin response in diabetic neuropathy. Muscle Nerve 10(1987)711-716.
24. SRINIVASAN, H., RAO, K. S. and SHANMUGAM, N. Steroid therapy in recent "quiet nerve paralysis" in leprosy. Lepr. India 54(1982)412-419.
25. TOUW-LANGENDUK, E. M. J., BRANDSMA, J. W. and ANDERSEN, J. G. Treatment of ulnar and median nerve function loss in borderline leprosy. Lepr. Rev. 55(1984)41-46.
26. VAN BRAKEL, W. H. Peripheral neuropathy in lep rosy. M.D. thesis. The Netherlands, 1994, pp. 95-114.
27. VAN BRAKEL, W. H. and KHAWAS, I. B. Nerve damage in leprosy; an epidemiological and clinical study of 396 patients in west Nepal-part I. Lepr. Rev. 65(1994) 204-221.
28. WILDER-SMITH, A. and WlLDER-SMITH, E. Electrophysiological evaluation of peripheral autonomic function in leprosy patients, leprosy contacts and controls. Int. J. Lepr. 64(1996)433-440.
29. WILDER-SMITH, E., WILDER-SMITH, A., VAN BRAKEL, W. II. and EGGER, M. Vasomotor rellex testing in leprosy patients, healthy contacts and controls: a cross-sectional study in western Nepal. Lepr. Rev. (in press).
30. WILDER-SMITH, E., WORPEL, F. and WILDER-SMITH, A. Changes of autonomic nerve function in the first 2 weeks of acute neuritis in a patient with borderline leprosy. (Letter) Int. J. Lepr. 64(1996)169-170.
1. M.D., D.T.M.&H., Medical Services International, P. O. Box 79511, Mongkok, Hong Kong.
2. M.D., D.T.M.&H., Neurological Department, University of Bern Inselspital. Bern CH-3010. Switzerland.
Received for publication on 8 July 1996; accepted for publication in revised form on 2 December 1996.