- Volume 65 , Number 1
- Page: 80–9
Cytokine gene expression in the foot pad and spleen of BALB/cAJcl mice infected with M. leprae
ABSTRACT
The cytokine mRNAs expressed in the foot pads and spleens of BALB/cAJcl mice infected with Mycobacterium leprae were studied by the reverse transcriptase-polymerase chain reaction (RT-PCR) method using cytokine-specific primers for interleukin-1 alpha (IL-1 α), -2, -4, -6, -10, -12(p40), gamma interferon (IFN-γ), tumor necrosis factor-alpha (TNF-α), and TNF- β and then for CD4 and CD8 markers. The pattern of cytokine gene expression in the foot pad which supports M. leprae growth was different f rom the expression in the spleen which does not permit M. leprae multiplication in mice. Before BALB/cAJcl mice were infected with M. leprae, IL-1α and TNF- β ; mRNAs were expressed physiologically in the foot pad while all of the cytokine genes examined were expressed in the spleen. In the foot pads of mice inoculated with M. leprae, in addition to the physiological appearance of IL-1 α and TNF- β mRNAs, these signals were intensified. TNF-α expression was induced by the infection. On the other hand, in the spleens of mice inoculated with M. leprae, CD4 mRNA expression disappeared on day 1 of the infection, which was accompanied by the reduced expression of IL-2, -4, -6, and -12 mRNAs. The recovery of CD4 mRNA expression at a latter stage was accompanied by a corresponding increase of the cytokine mRNA expression. It was suspected that these results might permit restricted growth of M. leprae in the foot pads of normal mice. Furthermore, our study suggests that tissue-specific, local, immunologic characteristics are important in M. leprae growth.RÉSUMÉ
Les ARNm de cytokine exprimés dans les coussinets plantaires et les rates de souris BALB/cAJcl infectées par Mycobacterium leprae ont été étudiés par la méthode de réaction réverse de transcriptase-polymerase en chaîne (RT-PCR), en utilisant des marqueurs spécifiques de cytokines pour l'interleukine-1 alpha (IL-1α), -2, -4, -6, -10, -12(p40), gamma interféron (IFN-γ), le facteur de nécrose tumorale alpha (FNT -α), et le FNT- β , et pour les marqueurs CD4 et CDS. Le mode d'expression du gène de cytokine dans le coussinet plantaire qui supporte la croissance de M. leprae était différent de l'expression dans la raie qui ne permet pas la multiplication de M. leprae chez la souris. Avant que les souris BALB/cAJcl ne soient infectées par M. leprae. les ARNm d'IL -1α et FNT- β ont été exprimés physiologiquement dans le coussinet plantaire alors que tous les gènes de cytokines examinés ont été exprimés dans la rate. Dans les coussinets plantaires des souris inoculéses avec M. leprae. en plus de l'apparition physiologique des ARNm d'IL-1α et FNT- β , ces signaux ont élé intensifiés. L'expression île FNT -α était induite par l'infection. D'autre part, dans les rates de souris infectées par M. leprae. l'expression de l'ARNm de CD4 disparut au premier jour de l'infection, qui était accompagnée d'une réduction de l'expression des ARNm d'IL-2, -4, -6 et -12. Le rétablissement de l'expression de l'ARNm de CD4 à un stade plus tardif était accompagné d'une augmentation correspondante de l'expression de l'ARNm de cytokine. On a soupçonné que ces résultats pourraient permettre une croissance limitée de M. leprae dans les coussinets plantaires de souris normales. De plus, notre étude suggère que les caractéristiques locales, spécifiques par tissu, sont importâmes pour la croissance de M. leprae.RESUMEN
Utilizando la reacción en cadena de la polimerasatranscriptasa reversa (RT-PCR) se estudiaron los mRNAs para citocinas expresados en las almohadillas plantares de ratones BALB/cAJcl infectados con Mycobacterium leprae. Se utilizaron sondas (iniciadores) para interleucina-l alfa (IL-α), IL-2, IL-4, IL-6, IL-10, IL-12 (p40), interferon gamma (IFN-γ), factor de necrosis tumoral alfa (TNFα), TNF β , para los marcadores CD4 y CD8. El patrón de expresión de los genes para citocinas en la almohadilla plantar (que permite el crecimiento de M. leprae) fue diferente del patrón de expresión de citocinas en el bazo (el cual no permite la multiplicación de M. leprae en el ratón). Mientras que sólo los mRNAs para IL-1α y TNF β se expresaron fisiológicamente en las almohadillas plantares de los ratones BALB/cAJCl antes de su infección con M, leprae, todos los genes para citocinas examinados fueron expresatlos en los bazos. En las almohadillas plantares de los ratones inoculados con M. leprae, además de la aparición fisiológica de los mRNAs para IL-1α y TNF β , estas señales fueron intensificadas. La expresión de TNFcr fue inducida por la infección. Por otro lado, en los bazos de los animales inoculados con M. leprae, la expresión del mRNA para Cl)4 desapareció hacia el día I tic la infección y ésto se acompañó de la expresión reducida de los mRNAs para 11.-2, II.-4, II.-6, e IL-12. La recuperación de la expresión del mRNA para CD4 en los estadios más avanzados tic la infección, se acompañó del correspondiente incremento en la expresión de los mRNAs para las otras citocinas. Estos resultados podrían estar relacionados con el crecimiento restringido de M. leprae en las almohadillas plantares de los ratones normales. Además, nuestro estudio sugiere que las características locales y específicas de tejido influyen importantemente en el crecimiento de M. leprae.The mechanisms of pathogenesis for Mycobacterium leprae infection remains unknown. Mice with cellular immunodeficiency are highly susceptible to M. leprae which is an intracellular pathogen (4,6,13,16,22,33,49,50). In immunocompetent mice, the only susceptible sites to M. leprae infection are skin tissues, such as the foot pad and the ear. When M, leprae are inoculated into the foot pads of normal mice, the bacilli can multiply in the site but the infection is limited to 107 bacilli (32,37-39). Even though M. leprae were inoculated into normal mice by intravenous injection, they were not able to multiply in the viscera such as the liver and the spleen (33). To elucidate the reasons for the restricted growth of M. leprae in the foot pads of immunocompetent mice, we examined and compared the expression of cytokine mRNAs in the foot pads and spleens of BALB/cAJcl mice infected with M. leprae.
MATERIALS AND METHODS
Mice. BALB/cAJcl mice were bred in the Central Institute for Experimental Animals, Kanagawa, Japan. Twenty female mice aged 6 weeks were used in the experiment. They were housed in the Animal Care Facility of our laboratory after inoculation with M. leprae.
M. leprae. Leprosy bacilli, Thai-53 strain, derived from foot pad passage of nude mice were used. The suspensions of viable (37) or heat-killed M. leprae (20) were prepared as described by references indicated.
Inoculation. Mice were infected with viable or heat-killed M. leprae each in both hind foot pads (2 x 107) combined with intravenous inoculation at a dose of 4 x 107. Two mice each were sacrificed on days 1, 10, 30 and 150 of inoculation, and the results were compared with those of the mice prior to infection (24 hr before infection; day 0).
Cytokine genes detection by RT-PCR. The expression of cytokine genes in the foot pads and spleens was examined by the reverse transcriptase-polymerase chain reaction (RT-PCR) method. RT-PCR of cytokine mRNAs from mouse tissues uninoculated (day 0) or inoculated with M. leprae at varying periods of time as described above was performed as described (47,48). In brief, total RNA was extracted by the acid guanidinium method from 2 foot pads and 2 spleens which were isolated from 2 infected mice, pooled and frozen, and cDNA was synthesized using Moloney murine leukemia virus (M-MLV) reverse transcriptase and oligo (dT). Reactions were incubated in a thermal cycler (Astec High Voltage, Ashland, Massachusetts, U.S.A.; PC-800) for 35 cycles. The cDNA concentrations were normalized to yield equivalent β -Actin PCR products. After PCR, 10 µ l of the DNA from each tube were loaded onto 1.5% agarose gels in TAE buffer. Products were visualized by ethidium bromide staining. The sense and antisense primers used were as follows: Interleukin-1 alpha (IL-1 α), 5 '-CTCTAG AGCACCATGCTACAGAC-3' and 5 '-TGGAATCCAGGGGAAACACTG-3'. IL-2, 5 '-ATGTACAGCATGCAGCTCGCATC-3' and 5 -GGCTTGTTGAGATGATGCTTTGACA-3'. IL-4, 5-A-TGGGTCTCAACCCCCAGCTAGT-3' and 5 '-GCTCTTTAGGCTTTCCAGG A AGTC3'. 1L-6, 5 '-ATG A AGTTCCTCTCTGC A-AGAGACT-3' and 5 '-CACTAGGTTTGC-CGAGTAGATCTC-3'. IL-10, 5'-TACC-TGGTAGAAGTGATGCC-3' and 5 '-CATCATGTATGCTTCTATGC-3'. IL-12 (p40), 5 '-CAG AAGCTAACCATCTGGTTTG-3' and 5 '-TCCGGAGTAATTTGGTGCTTCACAC-3'. Gamma interferon (IFN-γ), 5'-TGAACGCTACACACTGCATCTTGG3' and 5'-CGACTCCTTTTCCGCTTCCTGAG-3'. Tumor necrosis factor-alpha (TNF-α), 5'-GGCAGGTCTACTTTGG-AGTCATTGC-3' and 5 '-ACATTCGAG-GCTCCAGTGAATTCGG-3'. TNF- β , 5'-TGGCTGGGAACAGGGGAAGGTTGAC3' and 5'-CGTGCTTT CTTCTAG AAC-CCCTTGG-3'. CD4, 5'-TGTGCCGAGC-CATCTCTCTTAGG-3' and 5'-GCACTG-AGAGTGTCATGCCGAAC-3'. CD8, 5'-ATGCAGCCATGGCTCTGGCTGG-3' and 5 '-GCATGTCAGGCCCTTCTGGGTC-3'. 0-Actin, 5'-TGGAATCCTGTGG-CATCCATG A A AC-3' and 5 '-TAAAACG-CAGCTCAGTA ACAGTCCG-3'.
Immunohistologic staining of mouse foot pad tissues. Lymphocytes bearing CD4 and CD8 surface expression in the foot pad prior to the inoculation and 30 days after viable M. leprae inoculation were determined by immunohistochemistry. The tissue with OCT medium (Miles, Inc., Elkhart, Indiana, U.S.A.) was cut for 6- µ m thickness, fixed with acetone and chloroform, and then blocked with normal rabbit serum (Dako Corp., Carpenteria, California, U.S.A.; X902) before undergoing incubations with the monoclonal antibodies (anti-CD4 and anti-CD8; Serotec, Kidlington, U.K.; KT174 and KT15) overnight at 4ºC followed by biotinylated rabbit anti-rat immunoglobulins (Dako E0468) for 30 min. Slides were washed with phosphate buffered saline (PBS) between incubations. Primary antibodies were visualized by using the LSAB kit (Dako). Slides were counterstained with hematoxylin and mounted in Ukitt (O. Kinder, Germany).
ELISA. The mouse serum immunoglobulin of each group was measured by an ELISA method using goat anti-mouse IgG and IgM. The serum antibodies to M. leprae phenolic glycolipid-I (PGL-I), lipoarabinomannan-B (LAM-B) and heat-shock protein 65-kDa (hsp65 kDa) were measured by the ELISA method of Cho, et al. (5). LAM-B was prepared in our laboratory from a M. leprae cell-wall fraction obtained from armadillo tissues (11); PGL-I also was prepared from M. leprae -infected armadillo tissues using the method by Hunter, et al. ("'•12). M. Ieprae- hsp65 was also prepared in our laboratory from an Escherichia coli strain and affinity-purified with monoclonal antibody to hsp65 (26). Mouse serum was diluted at 1:100 with 2% bovine serum albumin-phosphate buffered saline-azide (BSA-PSA-azide) for antibody titration. The microplates (Nunc, Roskilde, Denmark) were coated with 50 µ l/well of PGL 1 at 2 µ g/ml, LAM-B at 1 µ g/ml, or hsp65 at 2 µ g/ml in 0.05 ml carbonate buffer (pH 9.6); the other half of each plate was coated with a coating buffer alone as a control. The peroxidase-conjugated goat anti-mouse IgG F(ab')2 and IgM F(ab')2 fragment (diluted 1:1000) was used for secondary antibody. Serum antibody level (OD 490 _ 650nm ) was calculated by subtracting the mean optical density (OD) of the control wells from the mean OD of the antigen-coated wells.
RESULTS
Cytokine mRNA expression in foot pads of M. leprae- inoculated mice. The changes in the cytokine mRNAs expression over time in the foot pads of uninoculated (24 hr before infection; day 0) and intravenously inoculated mice combined with the foot pad injection with viable or heatkilled M. leprae are shown in Figure 1. Physiologic expression of IL-1α and TNF- β mRNAs were observed in the foot pads of the uninoculated mice. In addition, in mice inoculated with viable M. leprae, the usual appearance of IL-la and TNF- β mRNA was intensified; TNF-α mRNA was induced on day 1 of inoculation. The expression of these mRNAs increased with the progression of the infection until day 10, and then decreased on day 30 and increased again on day 150. The same pattern of mRNA expression was also observed in mice inoculated with heat-killed M. leprae; the degree of induction of these mRNAs was lower on day 1 and the expression was slightly stronger after day 10.
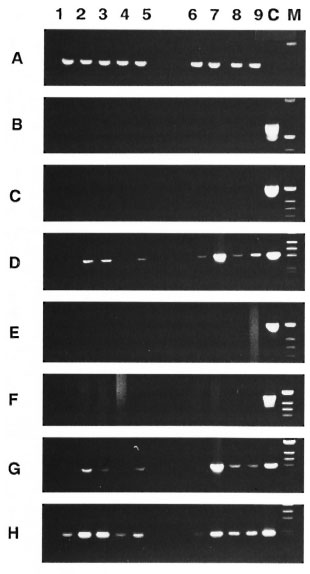
Fig 1. Cytokine mRNA expression in foot pads of M. leprae -inoculated mice. PCR analysis of cDNA from two foot pad tissues pooled from 2 infected micewas carried out at varying times by: lane 1, 24 hr before infection (day 0); lane 2, on day 1; lane 3. on day10; lane 4, on day 30: lane 5, on day 150 after infectionwith viable M. leprae. Lane 6, on day I; lane 7. on day10; lane 8, on day 30; lane 9, on day 150 after infectionwith heat-killed M. leprae. Lane C was the positivecontrol from ConA-stimulated spleen cells. Specificprimers used for the sequence of cytokines, CD4 andCD8 were : A = β -Actin, 13 = CD4, C = CD8, D = IL-
E = IL-2, F = G = TNF-α and H = TNF- β . Reactions were incubated in an Astec-800 for 35 cycles as conditions with denaturation 1 min, 94°C; annealing 2 min, 60°C or 65°C: extension 3 min, 72°C.
Cytokine mRNA expression in spleens of M. leprae -inoculated mice. The changes of cytokine mRNA expression in the spleen over time in uninoculated mice (day 0) and mice inoculated with viable or heat-killed M. leprae at varying periods of 1, 10, 30 and 150 days are shown in Figure 2. All of the cytokine mRNAs were examined; IL-1α, -2, -4, -6,-10, -12, IFN-γ, TNF-α and TNF- β were expressed physiologically in the spleens of uninoculated mice 24 hr before infection. CD4 and CD8 mRNAs also were expressed physiologically. In mice inoculated with viable M. leprae, CD4 mRNAs were undetectable on day 1 of inoculation, but the expression was recovered over time. In mice inoculated with heatkilled M. leprae, CD4 mRNA expression in the spleen was decreased up to day 30 of inoculation, and IL-2 and IL-6 mRNAs (except for a weak expression on day 1) were not expressed. An interesting observation was that the loss of CD4 mRNA expression on day I of inoculation of live M. leprae was accompanied by a diminished expression of IL-2, -4, -6 and IL-12 mRNAs, and the fluctuation in cytokine mRNA expression followed the pattern of fluctuation of CD4 mRNA expression. An increase in IL4 and IL-10 mRNAs expression was observed during the course of infection in mice inoculated with viable M. leprae. Fluctuation of IL-l-α mRNA expression was observed during the course of infection in mice inoculated with live bacteria but not in those inoculated with killed bacteria. TNF- β mRNA expression remained constant at the pre-inoculation level in both groups inoculated with viable and heatkilled M. leprae.
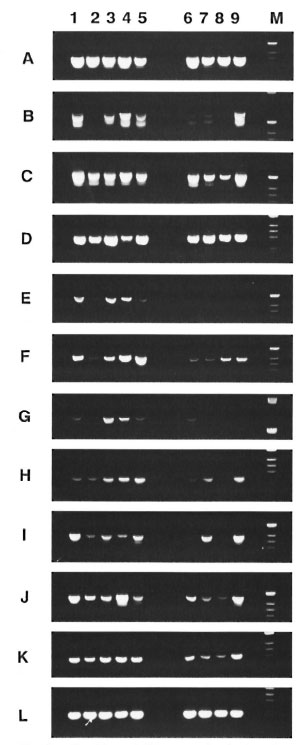
Fif. 2. Cytokine mRNA expression in the spleens of M. leprae -inoculated mice. PCR analysis of cDNA from 2 spleen tissues pooled from 2 infected mice werecarried out at varying times as well as the foot pad afterinoculation with viable or heat-killed M. leprae . Lane I ,24 hr before inoculation (day 01; lane 2, on day I; lane1 on day 10: lane 4, on day 30; lane 5, on day 150 after inoculation with viable M. leprae . Lane 6, on day Lane 7, on day 10: lane 8, on day 30: lane 9, on day 150 after inoculation with heat-killed M. leprae. Specific primers used were as follows: A = β -Actin. B = CD4, C= CD8, D = IL-1-α, F=IL-4, G = IL-6. H = = IL-12(p40), J = IFN-γ, K=TNF-α, L = TNF- β .
Immunohistological examinations of CD4+ and CD8+ lymphocytes in foot pads of M. leprae- infected mice. On day 30 of inoculation the foot pads received viable M. leprae and the foot pads prior to the inoculation were stained immunohistologically to examine for T-cell induction due to M. leprae infection. CD4+ and CD8+ lymphocytes were detected in the subcutaneous tissue and in the intermuscular layer as shown in Figure 3 but were not observed prior to the inoculation of M. leprae (data not shown).
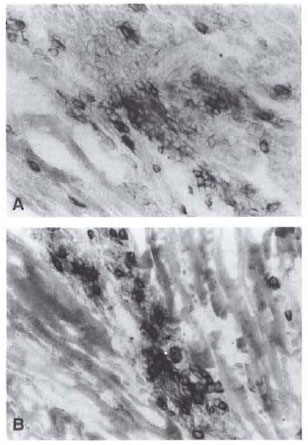
Fig. 3. Immunohistological stains of CD4 and CD8 lymphocytes in foot pads of a mouse on day 30 of infection with viahle M. leprae showing CD4+ (A) and CD8+ (B) lymphocyte infiltration into the intermuscu-ar layer (&Times;400).
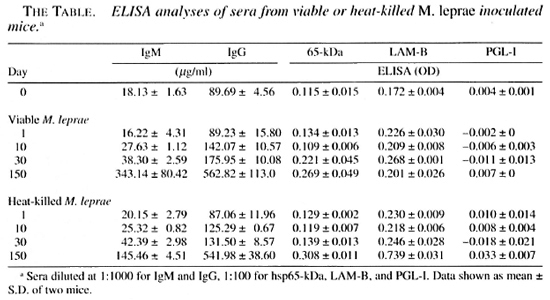
ELISA analyses of sera from M. leprae -inoculated mice. Increases in the IgM and IgG levels in the serum, due presumably to M. leprae inoculation, were observed 10 days after inoculation of M. leprae (The Table). There were no differences in increase of the antibody response to recombinant hsp65 between the groups infected with viable and heat-killed M. leprae. The antibody level to LAM-B antigen was evidently higher in the group inoculated with heat-killed M. leprae after 150 days of inoculation. No significant increases in antibody responses to PGL-I were observed in either group infected with viable or killed bacteria after 150 days of inoculation.
DISCUSSION
Since Hansen first described M. leprae as the pathogenic organism of leprosy, numerous animal experiments have been conducted (21,29). Shepard developed the foot pad inoculation method in mice and described that the low temperature in the foot pad has been an important factor for M. leprae growth even though the growth was limited (39,40) . We injected M. leprae into BALB/cAJcl mice intravenously and through foot pad roots combination, and studied the patterns of cytokine mRNA expression in the spleen and the foot pad of the mice with M. leprae by the RT-PCR method, extracting total RNA from these tissues, and synthesizing them into cDNA using a M-MLV reverse transcriptase. The patterns of cytokine mRNAs expressed in the foot pad tissue which permits the restricted growth of M. leprae were markedly different from that found in the spleen which does not allow M. leprae multiplication.
Kita, et al. (15) reported on the physiological expression of cytokine mRNAs in various organs of the BALB/cAJcl mouse. We could demonstrate a presence of patterns similar to Kita's in the spleen of mice prior to the inoculation (day 0), namely, all of the cytokine genes examined were found (Fig. 2). In the foot pads of mice, only the physiological presence of IL-l-α and TNF- β mRNA alone have been expressed (Fig. 1). Following the inoculation of viable or heatkilled M. leprae , the usual appearance of IL-l-a and TNF- β mRNA was intensified in the foot pad on day 1, and also the new expression of TNF-α mRNA was observed. In contrast to the fact that TNF-α is produced from activated various cells such as macrophages, TNF- β is considered to be produced from activated lymphocytes (28). In our results, the expression of mRNA for TNF- β but not TNF-α was detected in the foot pad prior to the inoculation of M. leprae without detectable mRNA for CD4+ and CD8+ cells. However, as seen in Figure 3, on day 30 following the foot pad inoculation of M leprae, the infiltration of CD4+ and CD8+ lymphocytes was confirmed by immunohistological staining. Here it is difficult to explain fully how a molecule can be expressed without a detectable mRNA, but it is considered that the number of infiltrated cells for CD4+ and CD8+ total number of cells in the foot pad may not be enough for the detection of the mRNA by the RT-PCR method. Further studies are necessary to elucidate the reasons.
The TNF-α was reported as a factor which induced hemorrhagic necrosis in the site of tumorigenesis (3), and it has begun to be construed as a cytokine closely related to defenses against pathogens (27-46') and tumors (30,44). In addition, IL-l-α, TNF-α and TNF- β possess similar biological activities and are known to exert synergism (8). In our experiments, the expressions of mRNA for these cytokines were observed to be stronger in the foot pads of mice following the inoculation of heat-killed M. leprae on day 10 than that seen in the foot pads following viable M. leprae inoculation, while the appearance of these cytokines was not changed in the spleens of mice inoculated with either viable or heat-killed M. leprae (Figs. 1 and 2).
The changes in the serum antibody titers of infected BALB/cAJcl mice were found from day 10 following the infection of M. leprae for the amount of IgM and IgG. and both of the specific antibodies against hsp65 kDa and LAM-B were found to increase when compared with titers prior to the infection. The titers of LAM-B were higher in the mice inoculated with heatkilled M. leprae than in those receiving viable bacteria on day 150 as shown in The Table. Although the elevation of the antibody level against PGL-1 was not observed, the presence of PGL-1 was confirmed by immunohistological stain in the tissues of viable M. leprae infection (data not shown). PGL-I (18,23,25,45) and LAM-B (41,42) are reported to be immunosuppressive antigens for cellular immunity, and hsp65 (24) is reported to be a highly immunogenic antigen for humoral and cellular immunity. When the foot pads of the BALB/cAJcl (euthymic) mice were inoculated with a large amount of M. leprae, the bacilli remain for a period of time in the inoculation site of the foot pad, demonstrating the establishment of persistent and resistant infection, even though there is no increase in the number of bacilli as reported by Rees (32). In the foot pad, cytokine production considered to eliminate the leprosy bacilli may be suppressed by PGL-I which is producing and releasing into tissues by viable M. leprae. In fact, PGL-I was detectable in the foot pad inoculated with M. leprae by immunohistological examination as mentioned above, and the antibody levels to LAM-B were evidently higher in the serum of the infected mice than those prior to the inoculation.
In contrast to the foot pad, all of the cytokine genes tested appeared in the spleen, except the disappearance of CD4 mRNA on day 1 of M. leprae infection accompanying the reduced expression of IL-2, -4, -6, and IL-12 mRNAs. The CD4 mRNA expression was recovered on day 10 of infection with viable M. leprae, with a corresponding increase in lymphokine mRNA expression which had been decreased on day 1. In other words, following the infection with M. leprae, the expression of CD4 mRNA is lost and reduced immediately; at the same time the reduction of the appearance of lymphokine genes occurs.
The results of the present study suggest that M. leprae exert a direct effect on CD4 lymphocytes which is related to inhibiting the expression of cytokine genes for the elimination of bacilli from the host temporarily. The temporary loss of expression of CD4 mRNA and the loss of accompanying cyktokine mRNA expressions in the spleen were stronger by the killed M. leprae inoculation than by the viable bacilli in the spleen. In the case of the killed bacilli inoculation, they never recovered up to 30 days of the post-inoculation, and the expression for IL-2 and IL-6 mRNAs never recovered, even the end of this experiment (Fig. 2). Based on these facts for cytokine patterns, in addition to the low temperature of the foot pad, as described by Shepard (39,40), it is considered that a long-term continuation specific to M. leprae infection is established in the foot pad.
Mitsuyama. et al. explained that killed bacteria have a weak ability to induce IL-1 production in macrophages so that T-cell activation cannot proceed, leading to a failure in eliciting delayed-type hypersensitivity (DTH) (19). In M. leprae infection, however, IL-1-or mRNA was detected in both the foot pads and the spleens of mice inoculated with killed M. leprae the same as viable M. leprae in our results shown in Figures 1 and 2. On day 10 after infection, the expression of IL-1-α mRNA was stronger in the foot pad by the killed M. leprae infection. It has been reported that the DTH reactions have been induced in mice pre-inoculated with killed (17) or viable M. leprae (17) by challenge with M. leprae lysate.
Recently, the analysis for the experimental results using TNF-α knockout mice clarified the role of this cytokine against intracellular parasites. When TNF-α p55 knockout mice were inoculated intravenously with Lysteria monocytogenes, all of the knockout mice died within 6 days after the inoculation while all of the normal control mice were alive (31,36). However, these knockout mice were normal against virus infection, showing normal function for cytotoxic T lymphocytes. There is a report that the administration of IL-l-α into mice inhibit the growth of intracellular parasites (7). In the intravenous inoculation of Lysteria, 90% of them are distributed in the liver, then killed and removed by Kupffer's cells. Thus, recent studies for the understanding of cellular parasitic infections indicated that nonspecific immunoregulation of macrophages is more important than the antigenspecific immune system by T lymphocytes (9,35). In the case of M. leprae which cannot multiply in the visceral organs, such as the spleen, similar removal mechanisms may also be established by the estimation of TNF-α mRNA patterns in our results (Figs. 1 and 2).
The cytokine genes, 1L-1-α, TNF-α and TNF- β mRNAs, which express in the foot pad of immunocompetent BALLVcAJcl mice infected with M. leprae were also observed in the foot pad of SCID and nude mice which are highly susceptible to M. leprae (51). It has been reported that IL-1 has the ability to promote the growth of pathogenic Escherichia coli (34). Beutler, et al. (2) have reported that a high degree of homology exists between the TNF and macrophage-secreted factor cachectin, and Kawakami and Hayata (14) have reported that TNF may be a potent cachexia or autoimmune inducing agent for the harmful excess of TNF production and, also, Amiri, et al. (1) have reported that schistosome utilizes TNF to maintain its progeny after oviposition. TNF- β also has been shown to be an important factor in the construction of lymph nodes (43). In the mechanisms of M. leprae infection and immunity, whether these cytokines play only a role in the elimination of M. leprae remains to be elucidated. In immunocompetent mice, the only sites that are susceptible to M. leprae infection are skin tissues, including the foot pad. Althoimh the significance of the expression of IL-1-α, TNF-α and TNF- β mRNAs in the foot pads of these mice cannot be ascertained without further studies, our present study suggests that tissue-specific, local, immunologic characteristics are important in M. leprae growth, and the overall immunologic competence of the infected host exerts further influence on its multiplication.
Acknowledgments. We thank Professor Roben L. Modlin and his staff, at U.CL.A., Los Angeles, California, U.S.A., for RT-PCR method. This work was supported by a grant from the Sasakawa Memorial I lealth Foundation.
REFERENCES
1. AMIRI, P.. LOCKSLEY, R. M, PARSLOW, T. G., SADICK, M., RECTOR, E., RITTER, D. and MCKER-ROW, J. H. Tumour necrosis factor a restores granulomas ami induces parasite egg-laying in schistosomes nfected SCID mice. Nature 356(1992)604-607.
2. BEUTLER, B., GREENWALD, D., HULMES, J. D., CHANG, M., PAN, Y.-C. E., MATHISON, J., ULEVITCH, R. and CERAMI, A. Identity of tumour necrosis factor and the macrophage-secreted factor cachectin. Nature 316(1985)552-554.
3. CARSWELL, E. A., OLD, L, J., KASSEL, R. I., ET AL. An endotoxin-induced serum factor that causes necrosis of tumors. Proc. Natl. Acad. Sci. U.S.A. 72(1975)3666-3670.
4. CHEHL, S., RUBY, J.. JOB, C. K. and HASTINGS, R. C. The growth of Mycobacterium leprae in nude mice. Lepr. Rev. 54(1983)283-304.
5. CHO, S.-N., YANAGIHARA, D. L., HUNTER, S. W., GELBER, R. H. and BRENNAN, P. J. Serological specificity of phenolic glycolipid-l from Mycobacterium leprae and use in serodiagnosis of leprosy. Infect. Immun. 41(1983)1077-1083.
6. COLSTON, M. J. AND HILSON, G. R. F. Growth of M. leprae and M. marinum in congenitally athymic (nude) mice. Nature 262(1976)399-401.
7. CZUPRYNSKI, C. J. anil BROWN, J. E. Recombinant murine interleukin-l a enhancement of nonspecific antibacterial resistance. Infect. Immun. 55(1987)2061-2065.
8. DINARELLO, C. A. Interleukin-l and its biological related cytokines. Adv. Immunol. 44(1989)153-205.
9. DREVETS, D. A., LEENEN, P. J. and CAMPBELL, P. A. Complement receptor type 3 (CD11b/CD 18) involvement is essential for killing of Listeria monocytogenes by mouse macrophages. J. Immunol. 151(1993)5431-5439.
10. HUNTER, S. W. anil BRENNAN, P. J. A novel phenolic glycolipid from Mycobacterium leprae possibly involved in immunogenicity and Pathogenicity. J. Bacterid. 147(1981)728-735.
11. HUNTER, S. W., GAYLORD, H. and BRENNAN, P. J. Structure and antigenicity of the phosphorylated lipopolysaccharide antigens from the leprosy and tubercle bacilli. J. Biol. Client. 261(1986)12345-12351.
12. HUNTER, S. W., STEWART. B. S. and BRENNAN, P. J. Purification of phenolic glycolipid I from armadillo and human sources. Int. J. Lepr. 53(1985)484-487.
13. ISHAQUE, M. Inoculation of foot pads of severe combined immunodeficient mice with M. leprae Int. J. Lepr. 60(1992)661.
14. KAWAKAMI, M. and HAYATA, K. [TNE] Med. Immunol. 20(1990)615-620.
15. KITA, M., TONG, L. J.,TANAKA, K. and IMANISHI, J. Expression de l'ARN messager des cytokines chez la souris dans des conditions physiologiques. C. R. Soc. Biol. 187(1993)414-419.
16. KOHSAKA, K., MORI, T. and ITOH, T. Lepromatoid lesion developed in nude mouse inoculated with M. leprae. Lepro 45(1976)177-187.
17. LAMB, F. I., KINGSTON, E., ESTRADA-G, I. and COLSTON, M. J. Heterologous expression of the 65-kilodulton antigen of Mycobacterium leprae and murine T-cell responses to the gene product. Infect. Immun. 56(1988)1237-1241.
18. MEHRA, V., BRENNAN, P. J., RADA, E., CONVIT, J. and BLOOM, B. R. Lymphocyte suppression in leprosy induced by unique M. leprae glycolipid. Nature 308(1984)194-196.
19. MITSDYAMA, M., IGARASHI, K., KAWAMURA, I., OHMORI, T. and NOMOTO, K. Difference in the induction of macrophage interleukin-1 production between viable and killed cells of Listeria monocytogenes. Am. Soc. Microbiol. 58(1990)1254-1260.
20. MITSUYAMA, M., WATANABE, M., SANO, K., AMAKO, K. and NOMOTO, K. Generation of Listeria monocytogenes-specific T cells mediating delayed foot pad reaction and protection in neonatal ly thymectomized mice but not in nude mice. Med. Microbiol. Immunol. 177(1988)207-217.
21. NAKAMURA, M. [Mycobacterium leprae and M. lepraemurium.] In: [Experimental Animals for Leprosy.] University of Tokai Press, Inc. (1985) Chapter 5, pp. 210-283.
22. NAKAMURA, K. and YOGI, Y. The nude mouse as an experimental lepromatous leprosy model: the enhancing effect of thymus cells in infected nude mice. (Abstract) Int. J. Lepr. Int. Suppl. (1979)338.
23. NEILL, M. A. and KLEBANOFF, S. J. The effect of phenolic clycolipid-1 from Mycobacterium leprae on the antimicrobial activity of human macrophages. J. Exp. Med. 167(1988)30-42.
24. NOMAGUCHI, H. [Heat shock protein and immunity.] Jpn. J. Lepr. 64(1995)188-199.
25. NOMAGUCHI, H., DOHI, Y , OHNO, N., ET AL. Suppression of ConA responses of mouse lymphocytes with unique M. leprae glycolipid. Jpn. J. Lepr. 58(1989)191-196.
26. NOMAGUCHI, H., MATSUOKA, M., KOHSAKA, K., NAKATA, A. and ITOH, T. Overproduction, affinity purification and characterization of 65-KDa protein of Mycobacterium leprae in Escherichia coli. Int. J. Lepr. 57(1989)817-824.
27. OLD, L. J. Tumor necrosis factor (TNF). Science 230(1985)630-632.
28. PAUL. N. L. and RUDDLE, N. H. Lymphotoxin. Annual Rev. Immunol. 6(1988)407-438.
29. PETER, A. S. and JOHNSTONE, M. A. The search for animal models of leprosy. Int. J. Lepr. 55(1987)535-547.
30. PHILIP, R. and EPSTEIN, L. B. Tumour necrosis factor as immunomodulator and mediator of monocyte cytotoxicity induced by itself, γ-interferon and interleukin-1. Nature 323(1986)86-89.
31. PLEFFER, K., MATSUYAMA, T., KUNDIG, T. M., ET AL. Mice deficient for the 55KD tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell 73(1993)457-467.
32. REES, R. J. W. Limited multiplication of acid-fast bacilli in the foot pads of mice inoculated with Mycobacterium leprae. Br. J. Exp. Pathol 45(1964)207-218.
33. REES, R. J. W., WATERS, M. F. R., WEDDELL, A. G. M. and PALMER, E. Experimental lepromatous leprosy. Nature 215(1967)599-602.
34. RORAT, R., CLARK, B. D., WOLFF, S. M. and DlNARELLO, C. A. Enhancement of growth of virulent strains of Escherichia coli by interleukin-1. Science 254( 1991)430-432.
35. ROSEN, H.,GORDON, S. and NORTH, R. J. Exacerbation of murine listeriosis by a monoclonal antibody specific for the type 3 complement receptor of myelo-monocytic cells. J. Exp. Med. 170(1989)27-37.
36. ROTHO, J., LESSLAUER, W., LOTSCHER, H., ET AL. Mice lacking the tumour necrosis factor receptor 1 are resistant to TNF-mediated toxicity but highly susceptible to infection by Listeria monocytogenes. Nature 364(1993)798-802.
37. SHEPARD, C. C. Acid-fast bacilli in nasal excretion in leprosy, and results of inoculation of mice. Am. J. Hyg. 71(1960)147-157.
38. SHEPARD, C. C. The experimental disease that follows the injection of human leprosy bacilli into foot pads of mice. J. Exp. Med. 112(1960)445-154.
39. SHEPARD, C. C. Multiplication of Mycobacterium leprae in the foot pad of the mouse. Int. J. Lepr. 30(1962)291-306.
40. SHEPARD, C. C. Considerations of the application of the foot pad technic in leprosy research. Int. J. Lepr. 33(1965)657-661.
41. SIBLEY, L. D., ADAMS, L. B. and KRAHENHUHL, J. L. Inhibition of interferon-gamma-mediated activation in mouse macrophages treated with lipoarabinomannan. Clin. Exp. Immunol. 80(1990)141-148.
42. SIBLEY, L. D., HUNTER, S. W., BRENNAN, P. J. and KRAHENBUHL, J. L. Mycobacterial lipoarabinomannan inhibits gamma-interferon-mediated activation of macrophages. Infect. Immun. 56(1988)1232-1236.
43. TOGNI, P. D., GOELLNER, J., RUDDLE, N. H., STREETER, P. R., FICK, A., MAKIATHASAN, S., SMITH, S. C, CARISON, R., SHORNICK, L. P., SCHOENBERGER, J. S., RUSSELL, J. H., KARR, R. and CHAPLIN, D. D. Abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin. Science 264(1994)703-707.
44. URBAN, J. L., SHEPARD, H. M., ROTHSTEIN, J. L. and SUGARMAN, B. J. Tumor necrosis factor: a potent effector molecule for tumor cell killing by activated macrophages. Proc. Natl. Acad. Sci. U.S.A. 83(1986)5233-5237.
45. VACHULA, M., HOLZER, T. J. and ANDERSEN, B. R. Suppression of monocyte oxidative response by phenolic glycolipid 1 of Mycobacterium leprae. J. Immunol. 142(1989)1696-1701.
46. WONG G. H. W. and GoEDDEL, D. V. Tumour necrosis factor α and β inhibit virus replication and cynergize with interferons. Nature 323(1986)819-822.
47. YAMAMURA, M., UYEMURA, K., DEANS, R. J., WEINBERG, K., REA, T. H., BLOOM, B. R. and MOULIN, R. L. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science 254(1991)277-279.
48. YAMAMURA, M., WANG, X.-H., OILMEN, J. D., UYEMURA, K., REA, T. H., BLOOM, B. R. and MOD-LIN, R. L. Cytokine patterns of immunologically mediated tissue damage. J. Immunol. 149(1992)1470-1475.
49. YOGI, Y, NAKAMURA, K., INOUE, T, KAWATSU, K., KASHIWABARA, Y, SAKAMOTO, Y, IZUMI, S., SAITO, M., HIOKI, K. and NONURA, T. Susceptibility of severe combined immunodeficient (SCID) mice to Mycobacterium leprae: multiplication of the bacillus and dissemination of the infection at early stage. Jpn. J. Lepr. 60(1991)139-145.
50. YOGI, Y, NAKAMURA, K. and SUZUKI, A. The experimental inoculation with Mycobacterium leprae in autoimmune mice: results of MRL /lpr mice inoculated into the right hind foot. Jpn. J. Lepr. 58(1989)235-240.
51. YOGI, Y, NOMAGUCHI, H., MATHUOKA, M., ET AL. [A study of cytokine mRNAs expression in inoculated mice with M. leprae .] Proc. Jpn. Soc. Immunol. 24(1994)554.
1. Ph.D., National Institute for Leprosy Research, 4-2-1 Aoba-cho, Higashimurayama-shi, Tokyo 189, Japan.
2. Ph.D., National Institute for Leprosy Research, 4-2-1 Aoba-cho, Higashimurayama-shi, Tokyo 189, Japan.
3. Ph.D., National Institute for Leprosy Research, 4-2-1 Aoba-cho, Higashimurayama-shi, Tokyo 189, Japan.
4. Ph.D., National Institute for Leprosy Research, 4-2-1 Aoba-cho, Higashimurayama-shi, Tokyo 189, Japan.
5. Ph.D., Department of Dermatology, Kitasato University, School of Medicine, 1-15-1 Kitasato, Sagamihara-shi, Kanagawa 228, Japan.
6. Ph.D., Department of Bacteriology, Hyogo College of Medicine, Nishinomiya, Hyogo 663, Japan.
7. B.S., Central Institute for Experimental Animals, 1430 Nogawa, Miyamae-ku, Kawasaki-shi, Kanagawa 216, Japan.
8. B.S., Central Institute for Experimental Animals, 1430 Nogawa, Miyamae-ku, Kawasaki-shi, Kanagawa 216, Japan.
9. M.D., Central Institute for Experimental Animals, 1430 Nogawa, Miyamae-ku, Kawasaki-shi, Kanagawa 216, Japan.
Received for publication on 18 September 1995.
Accepted for publication in revised form on 7 August 1996.